Iron Loses Electrons . If the oxidation number is positive, the atom loses electrons; Calcium has a charge of +2, indicating that it. Under the influence of the electric. Copper(ii) ions gain electrons and gain of electrons is reduction. In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). If the oxidation number is negative, the atom acquires electrons. This electron exchange is vital to the function of batteries, biological respiration, and even the principles behind many sensors and analytical techniques. This increases the oxidation state of the substance. The electronic configuration of iron is [ar] 3dx6 4sx2 [a r] 3 d x 6 4 s x 2. Magnesium loses electrons and loss of electrons is oxidation. In the anode, iron oxide loses electrons and forms fe 3+ ions that enter the molten salt. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. Oxidation is a chemical reaction where a substance loses electrons. It can lose two electrons to form the ferrous (fex2+) (f e x. One species loses electrons and another gains them.
from valenceelectrons.com
If the oxidation number is negative, the atom acquires electrons. It’s a chemical dance of give and take: It can lose two electrons to form the ferrous (fex2+) (f e x. In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). At its core, a redox reaction involves the transfer of electrons between two species. Copper(ii) ions gain electrons and gain of electrons is reduction. This increases the oxidation state of the substance. One species loses electrons and another gains them. The electronic configuration of iron is [ar] 3dx6 4sx2 [a r] 3 d x 6 4 s x 2. If the oxidation number is positive, the atom loses electrons;
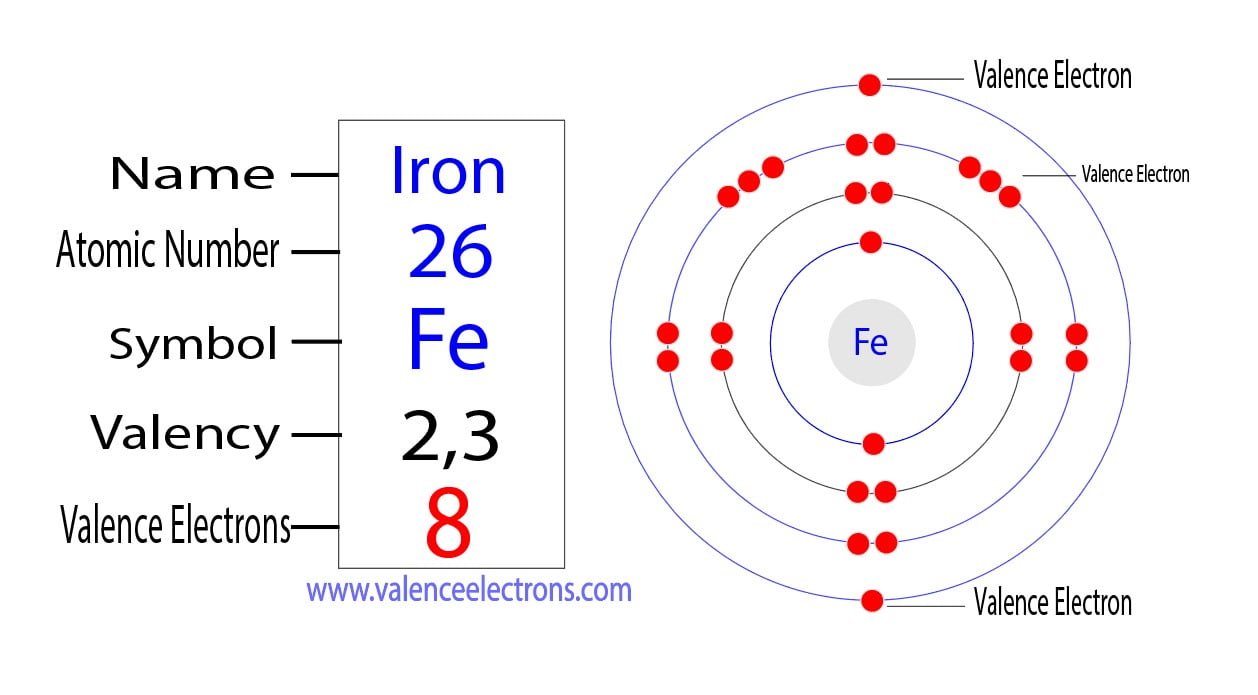
How Many Valence Electrons Does Iron (Fe) Have?
Iron Loses Electrons If the oxidation number is negative, the atom acquires electrons. Copper(ii) ions gain electrons and gain of electrons is reduction. In the anode, iron oxide loses electrons and forms fe 3+ ions that enter the molten salt. If the oxidation number is positive, the atom loses electrons; If the oxidation number is negative, the atom acquires electrons. One species loses electrons and another gains them. Oxidation is a chemical reaction where a substance loses electrons. Calcium has a charge of +2, indicating that it. It’s a chemical dance of give and take: In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). Under the influence of the electric. Magnesium loses electrons and loss of electrons is oxidation. This increases the oxidation state of the substance. This electron exchange is vital to the function of batteries, biological respiration, and even the principles behind many sensors and analytical techniques. The electronic configuration of iron is [ar] 3dx6 4sx2 [a r] 3 d x 6 4 s x 2. It can lose two electrons to form the ferrous (fex2+) (f e x.
From www.nagwa.com
Question Video Finding the Number of Electrons Lost or Gained by an Iron Loses Electrons Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. Oxidation is a chemical reaction where a substance loses electrons. This electron exchange is vital to the function of batteries, biological respiration, and even the principles behind many sensors and analytical techniques. One species loses electrons and another gains them.. Iron Loses Electrons.
From byjus.com
Iron loses its outermost 2 electrons of 4s2 orbital. Hence it has +2 Iron Loses Electrons It’s a chemical dance of give and take: Under the influence of the electric. It can lose two electrons to form the ferrous (fex2+) (f e x. In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). Magnesium loses electrons and loss of electrons is. Iron Loses Electrons.
From www.numerade.com
SOLVEDA zinc (Zn) atom loses two electrons to an ion. An iron Iron Loses Electrons It can lose two electrons to form the ferrous (fex2+) (f e x. At its core, a redox reaction involves the transfer of electrons between two species. The electronic configuration of iron is [ar] 3dx6 4sx2 [a r] 3 d x 6 4 s x 2. Under the influence of the electric. This increases the oxidation state of the substance.. Iron Loses Electrons.
From www.chegg.com
Solved Question 1 0.5 pts If a iron atom loses 2 electrons Iron Loses Electrons Under the influence of the electric. If the oxidation number is negative, the atom acquires electrons. In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). This increases the oxidation state of the substance. Copper(ii) ions gain electrons and gain of electrons is reduction. In. Iron Loses Electrons.
From www.slideserve.com
PPT Chemistry 120 PowerPoint Presentation, free download ID2089971 Iron Loses Electrons In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). If the oxidation number is positive, the atom loses electrons; One species loses electrons and another gains them. Magnesium loses electrons and loss of electrons is oxidation. Copper(ii) ions gain electrons and gain of electrons. Iron Loses Electrons.
From slideplayer.com
Bohr Diagrams Bohr diagrams show how many electrons appear in each Iron Loses Electrons It’s a chemical dance of give and take: Magnesium loses electrons and loss of electrons is oxidation. Calcium has a charge of +2, indicating that it. One species loses electrons and another gains them. It can lose two electrons to form the ferrous (fex2+) (f e x. Copper(ii) ions gain electrons and gain of electrons is reduction. This increases the. Iron Loses Electrons.
From slideplayer.com
Unit 2 Chemistry and Radioactivity ppt download Iron Loses Electrons If the oxidation number is negative, the atom acquires electrons. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. This increases the oxidation state of the substance. The electronic configuration of iron is [ar] 3dx6 4sx2 [a r] 3 d x 6 4 s x 2. If the oxidation. Iron Loses Electrons.
From www.chegg.com
Solved A Oxidation Compound A loses electrons A Oxidized Iron Loses Electrons Magnesium loses electrons and loss of electrons is oxidation. This electron exchange is vital to the function of batteries, biological respiration, and even the principles behind many sensors and analytical techniques. If the oxidation number is negative, the atom acquires electrons. At its core, a redox reaction involves the transfer of electrons between two species. This increases the oxidation state. Iron Loses Electrons.
From socratic.org
Question 587a9 Socratic Iron Loses Electrons It can lose two electrons to form the ferrous (fex2+) (f e x. Calcium has a charge of +2, indicating that it. In the anode, iron oxide loses electrons and forms fe 3+ ions that enter the molten salt. In order to write the iron electron configuration we first need to know the number of electrons for the fe atom. Iron Loses Electrons.
From slideplayer.com
Physical vs. Chemical Properties ppt download Iron Loses Electrons One species loses electrons and another gains them. It’s a chemical dance of give and take: This increases the oxidation state of the substance. In the anode, iron oxide loses electrons and forms fe 3+ ions that enter the molten salt. The electronic configuration of iron is [ar] 3dx6 4sx2 [a r] 3 d x 6 4 s x 2.. Iron Loses Electrons.
From physicalsciencetext.weebly.com
7.6 Ions Physical Science Iron Loses Electrons One species loses electrons and another gains them. Oxidation is a chemical reaction where a substance loses electrons. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. It can lose two electrons to form the ferrous (fex2+) (f e x. Magnesium loses electrons and loss of electrons is oxidation.. Iron Loses Electrons.
From material-properties.org
Iron Periodic Table and Atomic Properties Iron Loses Electrons Copper(ii) ions gain electrons and gain of electrons is reduction. If the oxidation number is positive, the atom loses electrons; Oxidation is a chemical reaction where a substance loses electrons. Magnesium loses electrons and loss of electrons is oxidation. In the anode, iron oxide loses electrons and forms fe 3+ ions that enter the molten salt. One species loses electrons. Iron Loses Electrons.
From www.numerade.com
SOLVED If an iron atom loses 3 electrons to make an ion what is the Iron Loses Electrons Magnesium loses electrons and loss of electrons is oxidation. Copper(ii) ions gain electrons and gain of electrons is reduction. This increases the oxidation state of the substance. Under the influence of the electric. If the oxidation number is positive, the atom loses electrons; It can lose two electrons to form the ferrous (fex2+) (f e x. One species loses electrons. Iron Loses Electrons.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Iron Have? Iron Loses Electrons Oxidation is a chemical reaction where a substance loses electrons. At its core, a redox reaction involves the transfer of electrons between two species. Magnesium loses electrons and loss of electrons is oxidation. Copper(ii) ions gain electrons and gain of electrons is reduction. One species loses electrons and another gains them. It’s a chemical dance of give and take: The. Iron Loses Electrons.
From guides.byjusweb.com
Electronic Configuration of Iron Fe element Valency, Applications Iron Loses Electrons If the oxidation number is negative, the atom acquires electrons. The electronic configuration of iron is [ar] 3dx6 4sx2 [a r] 3 d x 6 4 s x 2. This electron exchange is vital to the function of batteries, biological respiration, and even the principles behind many sensors and analytical techniques. If the oxidation number is positive, the atom loses. Iron Loses Electrons.
From www.youtube.com
Valence Electrons for Fe (Iron) YouTube Iron Loses Electrons In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). At its core, a redox reaction involves the transfer of electrons between two species. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. Magnesium. Iron Loses Electrons.
From slideplayer.com
OXIDATIONREDUCTION CHAPTER 19. OXIDATIONREDUCTION REACTIONS OXIDATION Iron Loses Electrons In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). If the oxidation number is positive, the atom loses electrons; Copper(ii) ions gain electrons and gain of electrons is reduction. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively. Iron Loses Electrons.
From farmerlextre.blogspot.com
Number Of Electrons In Iron Farmer Lextre Iron Loses Electrons Copper(ii) ions gain electrons and gain of electrons is reduction. This increases the oxidation state of the substance. The electronic configuration of iron is [ar] 3dx6 4sx2 [a r] 3 d x 6 4 s x 2. In the anode, iron oxide loses electrons and forms fe 3+ ions that enter the molten salt. If the oxidation number is negative,. Iron Loses Electrons.
From www.numerade.com
SOLVED What are the quantum numbers of the electrons that are lost by Iron Loses Electrons If the oxidation number is positive, the atom loses electrons; This increases the oxidation state of the substance. Copper(ii) ions gain electrons and gain of electrons is reduction. Calcium has a charge of +2, indicating that it. At its core, a redox reaction involves the transfer of electrons between two species. Under the influence of the electric. It can lose. Iron Loses Electrons.
From slideplayer.com
OXIDATION ANY REACTION IN WHICH A SUBSTANCE LOSES ELECTRONS ppt video Iron Loses Electrons Magnesium loses electrons and loss of electrons is oxidation. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. Oxidation is a chemical reaction where a substance loses electrons. If the oxidation number is positive, the atom loses electrons; Copper(ii) ions gain electrons and gain of electrons is reduction. In. Iron Loses Electrons.
From valenceelectrons.com
How Many Valence Electrons Does Iron (Fe) Have? Iron Loses Electrons Copper(ii) ions gain electrons and gain of electrons is reduction. If the oxidation number is negative, the atom acquires electrons. It’s a chemical dance of give and take: Under the influence of the electric. The electronic configuration of iron is [ar] 3dx6 4sx2 [a r] 3 d x 6 4 s x 2. One species loses electrons and another gains. Iron Loses Electrons.
From www.slideserve.com
PPT Oxidation an atom loses one or more electrons . PowerPoint Iron Loses Electrons This increases the oxidation state of the substance. Under the influence of the electric. In the anode, iron oxide loses electrons and forms fe 3+ ions that enter the molten salt. In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). It can lose two. Iron Loses Electrons.
From www.slideserve.com
PPT The Rusting of Iron PowerPoint Presentation, free download ID Iron Loses Electrons It’s a chemical dance of give and take: In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). The electronic configuration of iron is [ar] 3dx6 4sx2 [a r] 3 d x 6 4 s x 2. If the oxidation number is positive, the atom. Iron Loses Electrons.
From reviewhomedecor.co
Iron Periodic Table Electrons Review Home Decor Iron Loses Electrons Copper(ii) ions gain electrons and gain of electrons is reduction. In the anode, iron oxide loses electrons and forms fe 3+ ions that enter the molten salt. This increases the oxidation state of the substance. The electronic configuration of iron is [ar] 3dx6 4sx2 [a r] 3 d x 6 4 s x 2. It’s a chemical dance of give. Iron Loses Electrons.
From www.slideserve.com
PPT The Rusting of Iron PowerPoint Presentation, free download ID Iron Loses Electrons If the oxidation number is negative, the atom acquires electrons. Copper(ii) ions gain electrons and gain of electrons is reduction. If the oxidation number is positive, the atom loses electrons; The electronic configuration of iron is [ar] 3dx6 4sx2 [a r] 3 d x 6 4 s x 2. In the anode, iron oxide loses electrons and forms fe 3+. Iron Loses Electrons.
From www.youtube.com
How to find Protons & Electrons for Fe2+ and Fe3+ (Iron II and III ions Iron Loses Electrons The electronic configuration of iron is [ar] 3dx6 4sx2 [a r] 3 d x 6 4 s x 2. At its core, a redox reaction involves the transfer of electrons between two species. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. Copper(ii) ions gain electrons and gain of. Iron Loses Electrons.
From sarai-kobrien.blogspot.com
Metals Tend to Lose Electrons to Positive Ions Iron Loses Electrons In the anode, iron oxide loses electrons and forms fe 3+ ions that enter the molten salt. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. If the oxidation number is positive, the atom loses electrons; One species loses electrons and another gains them. It can lose two electrons. Iron Loses Electrons.
From www.istockphoto.com
Red Rust Is Caused By The Condition That Iron Loses Electrons When It Iron Loses Electrons Oxidation is a chemical reaction where a substance loses electrons. Calcium has a charge of +2, indicating that it. This electron exchange is vital to the function of batteries, biological respiration, and even the principles behind many sensors and analytical techniques. One species loses electrons and another gains them. The electronic configuration of iron is [ar] 3dx6 4sx2 [a r]. Iron Loses Electrons.
From www.chegg.com
Solved In the reaction of Fe with CuSO4, the iron a. loses Iron Loses Electrons Copper(ii) ions gain electrons and gain of electrons is reduction. This increases the oxidation state of the substance. Calcium has a charge of +2, indicating that it. The electronic configuration of iron is [ar] 3dx6 4sx2 [a r] 3 d x 6 4 s x 2. It can lose two electrons to form the ferrous (fex2+) (f e x. In. Iron Loses Electrons.
From www.nagwa.com
Question Video Determining What Forms When a Sodium Atom Loses One Iron Loses Electrons In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). The electronic configuration of iron is [ar] 3dx6 4sx2 [a r] 3 d x 6 4 s x 2. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged. Iron Loses Electrons.
From www.kgs.ku.edu
Untitled Document [www.kgs.ku.edu] Iron Loses Electrons Calcium has a charge of +2, indicating that it. In the anode, iron oxide loses electrons and forms fe 3+ ions that enter the molten salt. At its core, a redox reaction involves the transfer of electrons between two species. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the.. Iron Loses Electrons.
From www.slideserve.com
PPT RULES FOR ASSIGNING OXIDATION NUMBERS (STATES PowerPoint Iron Loses Electrons One species loses electrons and another gains them. It can lose two electrons to form the ferrous (fex2+) (f e x. Oxidation is a chemical reaction where a substance loses electrons. Copper(ii) ions gain electrons and gain of electrons is reduction. It’s a chemical dance of give and take: In the anode, iron oxide loses electrons and forms fe 3+. Iron Loses Electrons.
From valenceelectrons.com
How Many Valence Electrons Does Iron (Fe) Have? Iron Loses Electrons Oxidation is a chemical reaction where a substance loses electrons. The electronic configuration of iron is [ar] 3dx6 4sx2 [a r] 3 d x 6 4 s x 2. Calcium has a charge of +2, indicating that it. Copper(ii) ions gain electrons and gain of electrons is reduction. If the oxidation number is positive, the atom loses electrons; In the. Iron Loses Electrons.
From slideplayer.com
Chemical Reactions Chapter ppt download Iron Loses Electrons In the anode, iron oxide loses electrons and forms fe 3+ ions that enter the molten salt. Magnesium loses electrons and loss of electrons is oxidation. Copper(ii) ions gain electrons and gain of electrons is reduction. This electron exchange is vital to the function of batteries, biological respiration, and even the principles behind many sensors and analytical techniques. If the. Iron Loses Electrons.
From www.youtube.com
4.7 Ions Losing & Gaining Electrons YouTube Iron Loses Electrons At its core, a redox reaction involves the transfer of electrons between two species. One species loses electrons and another gains them. The electronic configuration of iron is [ar] 3dx6 4sx2 [a r] 3 d x 6 4 s x 2. Magnesium loses electrons and loss of electrons is oxidation. If the oxidation number is negative, the atom acquires electrons.. Iron Loses Electrons.