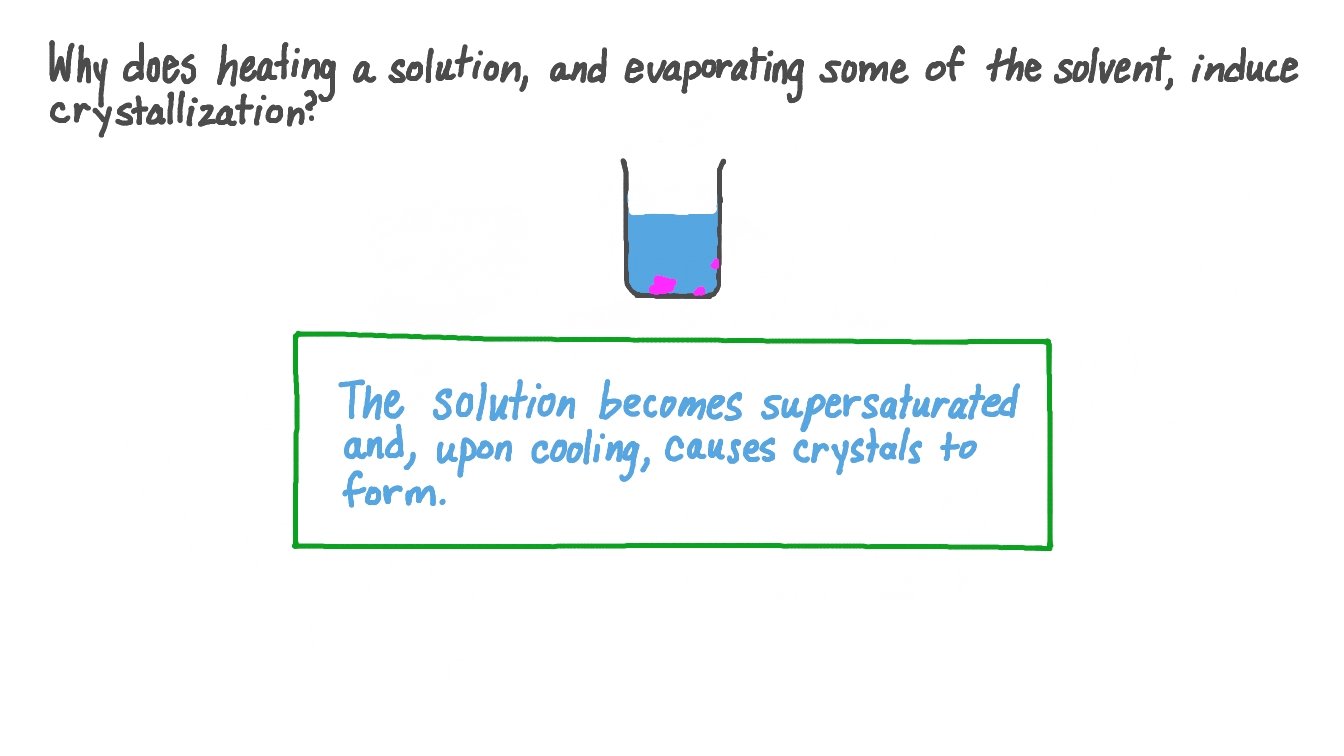
Why Do Crystals Form In Supersaturated Solutions . A supersaturated solution is a solution that contains more than the maximum amount of solute that is capable of being dissolved at a given. The reason why you can form a supersaturated solution at all is because the kinetics of crystallization are sometimes slow enough to. Exothermic crystallization from a supersaturated solution is demonstrated by pouring a solution of sodium acetate trihydrate onto crystals in a. Crystals tend to form slowly at room temperature, although you can knock sugar out of solution quickly if you refrigerate honey. Crystals grown by dissolving a solute in water rely on. These crystals provide the lattice structure. A supersaturated solution is unstable, and by seeding it you can trigger rapid crystallization. Sodium acetate can dissolve in water in. Once crystals start to form, their surface area increases as they grow. The excess solute starts to form crystals on the nuclei. In this case, a supersaturated solution of sodium acetate is poured over a crystal of sodium acetate. Crystals do not form unless the cooled solution is disturbed in some way, most often by allowing it to come into contact with a small crystalline fragment of the substance.
from www.nagwa.com
These crystals provide the lattice structure. Crystals grown by dissolving a solute in water rely on. In this case, a supersaturated solution of sodium acetate is poured over a crystal of sodium acetate. The excess solute starts to form crystals on the nuclei. A supersaturated solution is a solution that contains more than the maximum amount of solute that is capable of being dissolved at a given. A supersaturated solution is unstable, and by seeding it you can trigger rapid crystallization. The reason why you can form a supersaturated solution at all is because the kinetics of crystallization are sometimes slow enough to. Exothermic crystallization from a supersaturated solution is demonstrated by pouring a solution of sodium acetate trihydrate onto crystals in a. Crystals do not form unless the cooled solution is disturbed in some way, most often by allowing it to come into contact with a small crystalline fragment of the substance. Once crystals start to form, their surface area increases as they grow.
Question Video Explaining Why Heating a Solution Induces
Why Do Crystals Form In Supersaturated Solutions The reason why you can form a supersaturated solution at all is because the kinetics of crystallization are sometimes slow enough to. A supersaturated solution is a solution that contains more than the maximum amount of solute that is capable of being dissolved at a given. The reason why you can form a supersaturated solution at all is because the kinetics of crystallization are sometimes slow enough to. Once crystals start to form, their surface area increases as they grow. Crystals do not form unless the cooled solution is disturbed in some way, most often by allowing it to come into contact with a small crystalline fragment of the substance. Crystals tend to form slowly at room temperature, although you can knock sugar out of solution quickly if you refrigerate honey. In this case, a supersaturated solution of sodium acetate is poured over a crystal of sodium acetate. The excess solute starts to form crystals on the nuclei. Sodium acetate can dissolve in water in. These crystals provide the lattice structure. Crystals grown by dissolving a solute in water rely on. Exothermic crystallization from a supersaturated solution is demonstrated by pouring a solution of sodium acetate trihydrate onto crystals in a. A supersaturated solution is unstable, and by seeding it you can trigger rapid crystallization.
From fphoto.photoshelter.com
science chemistry solubility experiment supersaturation Fundamental Why Do Crystals Form In Supersaturated Solutions A supersaturated solution is unstable, and by seeding it you can trigger rapid crystallization. Exothermic crystallization from a supersaturated solution is demonstrated by pouring a solution of sodium acetate trihydrate onto crystals in a. Crystals do not form unless the cooled solution is disturbed in some way, most often by allowing it to come into contact with a small crystalline. Why Do Crystals Form In Supersaturated Solutions.
From preparatorychemistry.com
Supersaturation Why Do Crystals Form In Supersaturated Solutions Crystals grown by dissolving a solute in water rely on. These crystals provide the lattice structure. A supersaturated solution is unstable, and by seeding it you can trigger rapid crystallization. Exothermic crystallization from a supersaturated solution is demonstrated by pouring a solution of sodium acetate trihydrate onto crystals in a. Sodium acetate can dissolve in water in. Crystals do not. Why Do Crystals Form In Supersaturated Solutions.
From slideplayer.com
agitation) _____________ ppt download Why Do Crystals Form In Supersaturated Solutions Crystals tend to form slowly at room temperature, although you can knock sugar out of solution quickly if you refrigerate honey. A supersaturated solution is a solution that contains more than the maximum amount of solute that is capable of being dissolved at a given. These crystals provide the lattice structure. The excess solute starts to form crystals on the. Why Do Crystals Form In Supersaturated Solutions.
From www.studocu.com
Crystallization Saturated And Supersaturated Solutions, Crystal Why Do Crystals Form In Supersaturated Solutions Sodium acetate can dissolve in water in. Once crystals start to form, their surface area increases as they grow. In this case, a supersaturated solution of sodium acetate is poured over a crystal of sodium acetate. Crystals grown by dissolving a solute in water rely on. Exothermic crystallization from a supersaturated solution is demonstrated by pouring a solution of sodium. Why Do Crystals Form In Supersaturated Solutions.
From slideplayer.com
Secret lives of students HW checkVocab 22 Test results Notes ppt download Why Do Crystals Form In Supersaturated Solutions In this case, a supersaturated solution of sodium acetate is poured over a crystal of sodium acetate. A supersaturated solution is unstable, and by seeding it you can trigger rapid crystallization. Sodium acetate can dissolve in water in. Crystals do not form unless the cooled solution is disturbed in some way, most often by allowing it to come into contact. Why Do Crystals Form In Supersaturated Solutions.
From sciencenotes.org
Unsaturated Solution Definition and Examples in Chemistry Why Do Crystals Form In Supersaturated Solutions Sodium acetate can dissolve in water in. The excess solute starts to form crystals on the nuclei. Crystals do not form unless the cooled solution is disturbed in some way, most often by allowing it to come into contact with a small crystalline fragment of the substance. In this case, a supersaturated solution of sodium acetate is poured over a. Why Do Crystals Form In Supersaturated Solutions.
From fphoto.photoshelter.com
science chemistry solubility experiment supersaturation Fundamental Why Do Crystals Form In Supersaturated Solutions A supersaturated solution is a solution that contains more than the maximum amount of solute that is capable of being dissolved at a given. Exothermic crystallization from a supersaturated solution is demonstrated by pouring a solution of sodium acetate trihydrate onto crystals in a. These crystals provide the lattice structure. Crystals tend to form slowly at room temperature, although you. Why Do Crystals Form In Supersaturated Solutions.
From slideplayer.com
Solutions. ppt download Why Do Crystals Form In Supersaturated Solutions Exothermic crystallization from a supersaturated solution is demonstrated by pouring a solution of sodium acetate trihydrate onto crystals in a. These crystals provide the lattice structure. Once crystals start to form, their surface area increases as they grow. Crystals grown by dissolving a solute in water rely on. Sodium acetate can dissolve in water in. The excess solute starts to. Why Do Crystals Form In Supersaturated Solutions.
From www.pinterest.com
Supersaturated Solution Easy Science Chemistry education, Chemistry Why Do Crystals Form In Supersaturated Solutions Crystals tend to form slowly at room temperature, although you can knock sugar out of solution quickly if you refrigerate honey. Crystals grown by dissolving a solute in water rely on. The reason why you can form a supersaturated solution at all is because the kinetics of crystallization are sometimes slow enough to. Once crystals start to form, their surface. Why Do Crystals Form In Supersaturated Solutions.
From www.slideserve.com
PPT Solutions and Solubility PowerPoint Presentation, free download Why Do Crystals Form In Supersaturated Solutions Crystals do not form unless the cooled solution is disturbed in some way, most often by allowing it to come into contact with a small crystalline fragment of the substance. The reason why you can form a supersaturated solution at all is because the kinetics of crystallization are sometimes slow enough to. A supersaturated solution is a solution that contains. Why Do Crystals Form In Supersaturated Solutions.
From www.yaclass.in
Types of solutions Based on the amount of the solute — lesson. Science Why Do Crystals Form In Supersaturated Solutions Crystals tend to form slowly at room temperature, although you can knock sugar out of solution quickly if you refrigerate honey. The excess solute starts to form crystals on the nuclei. In this case, a supersaturated solution of sodium acetate is poured over a crystal of sodium acetate. Crystals do not form unless the cooled solution is disturbed in some. Why Do Crystals Form In Supersaturated Solutions.
From www.slideserve.com
PPT Solubility and Concentration PowerPoint Presentation, free Why Do Crystals Form In Supersaturated Solutions Once crystals start to form, their surface area increases as they grow. Crystals tend to form slowly at room temperature, although you can knock sugar out of solution quickly if you refrigerate honey. The excess solute starts to form crystals on the nuclei. A supersaturated solution is unstable, and by seeding it you can trigger rapid crystallization. Crystals do not. Why Do Crystals Form In Supersaturated Solutions.
From www.youtube.com
Supersaturated Sodium Acetate Solution YouTube Why Do Crystals Form In Supersaturated Solutions The excess solute starts to form crystals on the nuclei. Once crystals start to form, their surface area increases as they grow. The reason why you can form a supersaturated solution at all is because the kinetics of crystallization are sometimes slow enough to. Sodium acetate can dissolve in water in. These crystals provide the lattice structure. Exothermic crystallization from. Why Do Crystals Form In Supersaturated Solutions.
From chem.libretexts.org
13.2 Saturated Solutions and Solubility Chemistry LibreTexts Why Do Crystals Form In Supersaturated Solutions Sodium acetate can dissolve in water in. Crystals tend to form slowly at room temperature, although you can knock sugar out of solution quickly if you refrigerate honey. The excess solute starts to form crystals on the nuclei. A supersaturated solution is unstable, and by seeding it you can trigger rapid crystallization. A supersaturated solution is a solution that contains. Why Do Crystals Form In Supersaturated Solutions.
From www.slideshare.net
5.5.1 Why Do Crystals Form In Supersaturated Solutions Crystals tend to form slowly at room temperature, although you can knock sugar out of solution quickly if you refrigerate honey. These crystals provide the lattice structure. Crystals grown by dissolving a solute in water rely on. Sodium acetate can dissolve in water in. A supersaturated solution is a solution that contains more than the maximum amount of solute that. Why Do Crystals Form In Supersaturated Solutions.
From ar.inspiredpencil.com
Types Of Solutions Saturated Unsaturated And Supersaturated Why Do Crystals Form In Supersaturated Solutions The reason why you can form a supersaturated solution at all is because the kinetics of crystallization are sometimes slow enough to. The excess solute starts to form crystals on the nuclei. In this case, a supersaturated solution of sodium acetate is poured over a crystal of sodium acetate. Exothermic crystallization from a supersaturated solution is demonstrated by pouring a. Why Do Crystals Form In Supersaturated Solutions.
From exoknirsw.blob.core.windows.net
Why Do Crystals Form Geometric Shapes at Joshua Downs blog Why Do Crystals Form In Supersaturated Solutions Once crystals start to form, their surface area increases as they grow. The excess solute starts to form crystals on the nuclei. A supersaturated solution is unstable, and by seeding it you can trigger rapid crystallization. In this case, a supersaturated solution of sodium acetate is poured over a crystal of sodium acetate. A supersaturated solution is a solution that. Why Do Crystals Form In Supersaturated Solutions.
From slideplayer.com
Why is it harmful to pee in the pool? ppt download Why Do Crystals Form In Supersaturated Solutions These crystals provide the lattice structure. Crystals do not form unless the cooled solution is disturbed in some way, most often by allowing it to come into contact with a small crystalline fragment of the substance. Once crystals start to form, their surface area increases as they grow. Sodium acetate can dissolve in water in. The excess solute starts to. Why Do Crystals Form In Supersaturated Solutions.
From quizizz.com
M2 Solution part 1 Chemistry Quizizz Why Do Crystals Form In Supersaturated Solutions Sodium acetate can dissolve in water in. Crystals do not form unless the cooled solution is disturbed in some way, most often by allowing it to come into contact with a small crystalline fragment of the substance. Once crystals start to form, their surface area increases as they grow. In this case, a supersaturated solution of sodium acetate is poured. Why Do Crystals Form In Supersaturated Solutions.
From slideplayer.com
Figure Title Dissolution of an ionic solid in water. Caption ppt Why Do Crystals Form In Supersaturated Solutions These crystals provide the lattice structure. Crystals grown by dissolving a solute in water rely on. Once crystals start to form, their surface area increases as they grow. Crystals do not form unless the cooled solution is disturbed in some way, most often by allowing it to come into contact with a small crystalline fragment of the substance. A supersaturated. Why Do Crystals Form In Supersaturated Solutions.
From slideplayer.com
Water Properties REVIEW FOR QUIZ ppt download Why Do Crystals Form In Supersaturated Solutions Crystals grown by dissolving a solute in water rely on. The reason why you can form a supersaturated solution at all is because the kinetics of crystallization are sometimes slow enough to. Exothermic crystallization from a supersaturated solution is demonstrated by pouring a solution of sodium acetate trihydrate onto crystals in a. The excess solute starts to form crystals on. Why Do Crystals Form In Supersaturated Solutions.
From www.chemicalslearning.com
Supersaturation Crystallization Processes Methods of Supersaturation Why Do Crystals Form In Supersaturated Solutions Sodium acetate can dissolve in water in. Exothermic crystallization from a supersaturated solution is demonstrated by pouring a solution of sodium acetate trihydrate onto crystals in a. A supersaturated solution is unstable, and by seeding it you can trigger rapid crystallization. Once crystals start to form, their surface area increases as they grow. In this case, a supersaturated solution of. Why Do Crystals Form In Supersaturated Solutions.
From fphoto.photoshelter.com
science chemistry solubility experiment supersaturation Fundamental Why Do Crystals Form In Supersaturated Solutions The excess solute starts to form crystals on the nuclei. The reason why you can form a supersaturated solution at all is because the kinetics of crystallization are sometimes slow enough to. Crystals tend to form slowly at room temperature, although you can knock sugar out of solution quickly if you refrigerate honey. Exothermic crystallization from a supersaturated solution is. Why Do Crystals Form In Supersaturated Solutions.
From www.youtube.com
INTRODUCTION TO CRYSTALLIZATION FROM A SOLUTION YouTube Why Do Crystals Form In Supersaturated Solutions Crystals tend to form slowly at room temperature, although you can knock sugar out of solution quickly if you refrigerate honey. In this case, a supersaturated solution of sodium acetate is poured over a crystal of sodium acetate. Exothermic crystallization from a supersaturated solution is demonstrated by pouring a solution of sodium acetate trihydrate onto crystals in a. The excess. Why Do Crystals Form In Supersaturated Solutions.
From www.mdpi.com
Crystals Free FullText Molecular Mechanism of Organic Crystal Why Do Crystals Form In Supersaturated Solutions In this case, a supersaturated solution of sodium acetate is poured over a crystal of sodium acetate. A supersaturated solution is unstable, and by seeding it you can trigger rapid crystallization. Crystals do not form unless the cooled solution is disturbed in some way, most often by allowing it to come into contact with a small crystalline fragment of the. Why Do Crystals Form In Supersaturated Solutions.
From www.nagwa.com
Question Video Explaining Why Heating a Solution Induces Why Do Crystals Form In Supersaturated Solutions Once crystals start to form, their surface area increases as they grow. These crystals provide the lattice structure. Crystals grown by dissolving a solute in water rely on. Sodium acetate can dissolve in water in. Crystals tend to form slowly at room temperature, although you can knock sugar out of solution quickly if you refrigerate honey. Exothermic crystallization from a. Why Do Crystals Form In Supersaturated Solutions.
From www.revisechemistry.uk
Types of chemical reactions OCR Gateway C3 revisechemistry.uk Why Do Crystals Form In Supersaturated Solutions Sodium acetate can dissolve in water in. In this case, a supersaturated solution of sodium acetate is poured over a crystal of sodium acetate. Crystals do not form unless the cooled solution is disturbed in some way, most often by allowing it to come into contact with a small crystalline fragment of the substance. Once crystals start to form, their. Why Do Crystals Form In Supersaturated Solutions.
From sciencenotes.org
How to Grow Blue Copper Sulfate Crystals Why Do Crystals Form In Supersaturated Solutions Crystals do not form unless the cooled solution is disturbed in some way, most often by allowing it to come into contact with a small crystalline fragment of the substance. The reason why you can form a supersaturated solution at all is because the kinetics of crystallization are sometimes slow enough to. The excess solute starts to form crystals on. Why Do Crystals Form In Supersaturated Solutions.
From www.medicalimages.com
STOCK IMAGE, example of a supersaturated solution one that contains Why Do Crystals Form In Supersaturated Solutions In this case, a supersaturated solution of sodium acetate is poured over a crystal of sodium acetate. A supersaturated solution is unstable, and by seeding it you can trigger rapid crystallization. These crystals provide the lattice structure. Once crystals start to form, their surface area increases as they grow. The excess solute starts to form crystals on the nuclei. The. Why Do Crystals Form In Supersaturated Solutions.
From slideplayer.com
Solubility. ppt download Why Do Crystals Form In Supersaturated Solutions Exothermic crystallization from a supersaturated solution is demonstrated by pouring a solution of sodium acetate trihydrate onto crystals in a. Crystals tend to form slowly at room temperature, although you can knock sugar out of solution quickly if you refrigerate honey. Once crystals start to form, their surface area increases as they grow. These crystals provide the lattice structure. The. Why Do Crystals Form In Supersaturated Solutions.
From fphoto.photoshelter.com
science chemistry solubility experiment supersaturation Fundamental Why Do Crystals Form In Supersaturated Solutions Exothermic crystallization from a supersaturated solution is demonstrated by pouring a solution of sodium acetate trihydrate onto crystals in a. A supersaturated solution is a solution that contains more than the maximum amount of solute that is capable of being dissolved at a given. A supersaturated solution is unstable, and by seeding it you can trigger rapid crystallization. Crystals tend. Why Do Crystals Form In Supersaturated Solutions.
From fphoto.photoshelter.com
science chemistry solubility experiment supersaturation Fundamental Why Do Crystals Form In Supersaturated Solutions A supersaturated solution is unstable, and by seeding it you can trigger rapid crystallization. These crystals provide the lattice structure. Exothermic crystallization from a supersaturated solution is demonstrated by pouring a solution of sodium acetate trihydrate onto crystals in a. Crystals grown by dissolving a solute in water rely on. The excess solute starts to form crystals on the nuclei.. Why Do Crystals Form In Supersaturated Solutions.
From www.youtube.com
Hot Ice (HD) Crystals (Sodium Acetate) YouTube Why Do Crystals Form In Supersaturated Solutions Exothermic crystallization from a supersaturated solution is demonstrated by pouring a solution of sodium acetate trihydrate onto crystals in a. The excess solute starts to form crystals on the nuclei. Once crystals start to form, their surface area increases as they grow. A supersaturated solution is unstable, and by seeding it you can trigger rapid crystallization. Crystals grown by dissolving. Why Do Crystals Form In Supersaturated Solutions.
From www.syrris.com
Learn more about Crystallization by Syrris Why Do Crystals Form In Supersaturated Solutions The excess solute starts to form crystals on the nuclei. These crystals provide the lattice structure. Sodium acetate can dissolve in water in. Once crystals start to form, their surface area increases as they grow. In this case, a supersaturated solution of sodium acetate is poured over a crystal of sodium acetate. Crystals tend to form slowly at room temperature,. Why Do Crystals Form In Supersaturated Solutions.
From sciencenotes.org
Supersaturated Solution Definition and Examples Why Do Crystals Form In Supersaturated Solutions The excess solute starts to form crystals on the nuclei. These crystals provide the lattice structure. Sodium acetate can dissolve in water in. Crystals tend to form slowly at room temperature, although you can knock sugar out of solution quickly if you refrigerate honey. In this case, a supersaturated solution of sodium acetate is poured over a crystal of sodium. Why Do Crystals Form In Supersaturated Solutions.