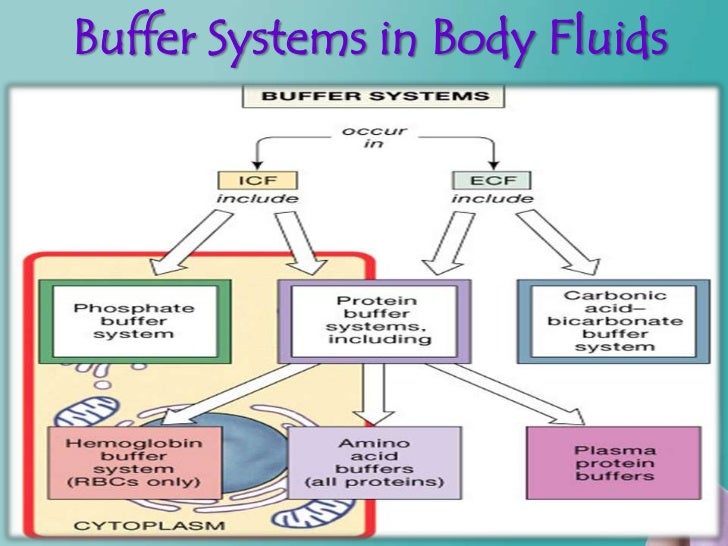
Phosphate Buffer In The Body . The phosphate buffer system is not an important blood buffer as its concentration is too low. All of these contain bases that accept hydrogen. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. Phosphates are found in the blood in two forms: Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. A variety of buffering systems exist in the body that helps maintain the ph of the blood and other fluids within a narrow. Phosphates are found in the blood in two forms: The concentration of phosphate in the blood is so. The phosphate buffer system is a crucial mechanism for regulating the ph of bodily fluids, particularly in the kidneys. Phosphates are found in the blood in two forms: Sodium dihydrogen phosphate (na h 2 po 4 na h 2 po 4), which is a weak. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and sodium monohydrogen phosphate (na.
from www.slideshare.net
The phosphate buffer system is a crucial mechanism for regulating the ph of bodily fluids, particularly in the kidneys. The concentration of phosphate in the blood is so. All of these contain bases that accept hydrogen. Phosphates are found in the blood in two forms: The phosphate buffer system is not an important blood buffer as its concentration is too low. Sodium dihydrogen phosphate (na h 2 po 4 na h 2 po 4), which is a weak. Phosphates are found in the blood in two forms: A variety of buffering systems exist in the body that helps maintain the ph of the blood and other fluids within a narrow. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and sodium monohydrogen phosphate (na.
Buffer system
Phosphate Buffer In The Body A variety of buffering systems exist in the body that helps maintain the ph of the blood and other fluids within a narrow. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. Phosphates are found in the blood in two forms: Phosphates are found in the blood in two forms: A variety of buffering systems exist in the body that helps maintain the ph of the blood and other fluids within a narrow. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and sodium monohydrogen phosphate (na. The concentration of phosphate in the blood is so. The phosphate buffer system is not an important blood buffer as its concentration is too low. The phosphate buffer system is a crucial mechanism for regulating the ph of bodily fluids, particularly in the kidneys. Phosphates are found in the blood in two forms: All of these contain bases that accept hydrogen. Sodium dihydrogen phosphate (na h 2 po 4 na h 2 po 4), which is a weak.
From doctorlib.info
Acidbase Balance Physiology An Illustrated Review Phosphate Buffer In The Body All of these contain bases that accept hydrogen. Phosphates are found in the blood in two forms: Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. The phosphate buffer system is not an important blood buffer as its concentration is too low. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which. Phosphate Buffer In The Body.
From www.slideserve.com
PPT Chapter 24 Water, Electrolyte and AcidBase Balance PowerPoint Phosphate Buffer In The Body Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and sodium monohydrogen phosphate (na. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. The phosphate buffer system is not an important blood buffer as its concentration is too low. A variety of buffering systems exist in the. Phosphate Buffer In The Body.
From ar.inspiredpencil.com
Phosphate Buffer System Equation Phosphate Buffer In The Body The phosphate buffer system is not an important blood buffer as its concentration is too low. The concentration of phosphate in the blood is so. Phosphates are found in the blood in two forms: A variety of buffering systems exist in the body that helps maintain the ph of the blood and other fluids within a narrow. All of these. Phosphate Buffer In The Body.
From animalia-life.club
Phosphate Buffer System Equation Phosphate Buffer In The Body All of these contain bases that accept hydrogen. Sodium dihydrogen phosphate (na h 2 po 4 na h 2 po 4), which is a weak. Phosphates are found in the blood in two forms: Phosphates are found in the blood in two forms: The phosphate buffer system is not an important blood buffer as its concentration is too low. The. Phosphate Buffer In The Body.
From www.pinterest.com
Physiology Blood Buffer System Behrouz Human body facts Phosphate Buffer In The Body All of these contain bases that accept hydrogen. Phosphates are found in the blood in two forms: Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. The concentration of phosphate in the blood is so. Phosphates are found in the blood in two forms: A variety of buffering systems exist in the body. Phosphate Buffer In The Body.
From www.slideserve.com
PPT Renal Physiology PowerPoint Presentation, free download ID5632772 Phosphate Buffer In The Body Phosphates are found in the blood in two forms: The phosphate buffer system is a crucial mechanism for regulating the ph of bodily fluids, particularly in the kidneys. Sodium dihydrogen phosphate (na h 2 po 4 na h 2 po 4), which is a weak. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid,. Phosphate Buffer In The Body.
From www.sliderbase.com
Acid and Base Balance and Imbalance Presentation Chemistry Phosphate Buffer In The Body Phosphates are found in the blood in two forms: Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and sodium monohydrogen phosphate (na. Phosphates are found in the blood in two forms: The concentration of phosphate in the. Phosphate Buffer In The Body.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Phosphate Buffer In The Body The phosphate buffer system is a crucial mechanism for regulating the ph of bodily fluids, particularly in the kidneys. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and sodium monohydrogen phosphate (na. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. Phosphates are found in the blood. Phosphate Buffer In The Body.
From pressbooks.bccampus.ca
26.4 AcidBase Balance Douglas College Human Anatomy and Physiology Phosphate Buffer In The Body Sodium dihydrogen phosphate (na h 2 po 4 na h 2 po 4), which is a weak. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. The phosphate buffer system is a crucial mechanism for regulating the ph. Phosphate Buffer In The Body.
From www.slideserve.com
PPT AcidBase Balance PowerPoint Presentation, free download ID1994730 Phosphate Buffer In The Body Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. Phosphates are found in the blood in two forms: The concentration of phosphate in the blood is so. The phosphate buffer system is not an important blood buffer as its concentration is too low. Other buffer systems in the human body include the phosphate. Phosphate Buffer In The Body.
From www.anaesthesiajournal.co.uk
Renal physiology acidbase balance Anaesthesia & Intensive Care Medicine Phosphate Buffer In The Body Phosphates are found in the blood in two forms: All of these contain bases that accept hydrogen. Phosphates are found in the blood in two forms: The phosphate buffer system is a crucial mechanism for regulating the ph of bodily fluids, particularly in the kidneys. A variety of buffering systems exist in the body that helps maintain the ph of. Phosphate Buffer In The Body.
From www.slideserve.com
PPT Fluid, Electrolyte, and AcidBase Balance PowerPoint Presentation Phosphate Buffer In The Body The phosphate buffer system is not an important blood buffer as its concentration is too low. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. Phosphates are found in the blood in two forms: Phosphates are found in the blood in two forms: Sodium dihydrogen phosphate (na h 2 po 4 na h. Phosphate Buffer In The Body.
From www.youtube.com
Phosphate forms (names, pKa's, etc.), buffers basics, & intuition YouTube Phosphate Buffer In The Body A variety of buffering systems exist in the body that helps maintain the ph of the blood and other fluids within a narrow. Phosphates are found in the blood in two forms: Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. The phosphate buffer system is a crucial mechanism for regulating the ph. Phosphate Buffer In The Body.
From www.slideserve.com
PPT AcidBase Equilibrium (Mono and Polyprotic) [Chapter 10,11 Phosphate Buffer In The Body Phosphates are found in the blood in two forms: A variety of buffering systems exist in the body that helps maintain the ph of the blood and other fluids within a narrow. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. The phosphate buffer system is a crucial mechanism for regulating the ph of. Phosphate Buffer In The Body.
From animalia-life.club
Phosphate Buffer System Equation Phosphate Buffer In The Body The concentration of phosphate in the blood is so. Phosphates are found in the blood in two forms: Phosphates are found in the blood in two forms: Sodium dihydrogen phosphate (na h 2 po 4 na h 2 po 4), which is a weak. Phosphates are found in the blood in two forms: Sodium dihydrogen phosphate (na 2 h 2. Phosphate Buffer In The Body.
From www.slideserve.com
PPT Unit Five The Body Fluids and Kidneys PowerPoint Presentation Phosphate Buffer In The Body Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. All of these contain bases that accept hydrogen. Phosphates are found in the blood in two forms: Phosphates are found in the blood in two forms: The phosphate buffer system is not an important blood buffer as its concentration is too low. Sodium dihydrogen phosphate. Phosphate Buffer In The Body.
From www.slideserve.com
PPT Buffer Systems of the Body PowerPoint Presentation, free download Phosphate Buffer In The Body The phosphate buffer system is not an important blood buffer as its concentration is too low. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and sodium monohydrogen phosphate (na. A variety of buffering systems exist in the body that helps maintain the ph of the blood and other fluids within a narrow. All. Phosphate Buffer In The Body.
From labpedia.net
Phosphorus (P), Phosphate (PO4), Phosphorus Phosphate Buffer In The Body Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and sodium monohydrogen phosphate (na. Phosphates are found in the blood in two forms: Phosphates are found in the blood in two forms: All of these contain bases that accept hydrogen. Other buffer systems in the human body include the phosphate buffer system, proteins, and. Phosphate Buffer In The Body.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID Phosphate Buffer In The Body Phosphates are found in the blood in two forms: A variety of buffering systems exist in the body that helps maintain the ph of the blood and other fluids within a narrow. The phosphate buffer system is a crucial mechanism for regulating the ph of bodily fluids, particularly in the kidneys. The phosphate buffer system is not an important blood. Phosphate Buffer In The Body.
From animalia-life.club
Phosphate Buffer System Equation Phosphate Buffer In The Body A variety of buffering systems exist in the body that helps maintain the ph of the blood and other fluids within a narrow. Sodium dihydrogen phosphate (na h 2 po 4 na h 2 po 4), which is a weak. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. The phosphate buffer system. Phosphate Buffer In The Body.
From www.youtube.com
Chemical Buffers protein buffer, phosphate buffer system and Phosphate Buffer In The Body Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. Phosphates are found in the blood in two forms: Phosphates are found in the blood in two forms: Sodium dihydrogen phosphate (na h 2 po 4 na h 2 po 4), which is a weak. Other buffer systems in the human body include the. Phosphate Buffer In The Body.
From www.slideserve.com
PPT Fluid, Electrolyte, and AcidBase Balance PowerPoint Presentation Phosphate Buffer In The Body Sodium dihydrogen phosphate (na h 2 po 4 na h 2 po 4), which is a weak. Phosphates are found in the blood in two forms: Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and sodium monohydrogen phosphate. Phosphate Buffer In The Body.
From www.vrogue.co
Phosphate Buffer System Equation vrogue.co Phosphate Buffer In The Body All of these contain bases that accept hydrogen. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. The phosphate buffer system is not an important blood buffer as its concentration is too low. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and sodium monohydrogen phosphate (na. Sodium. Phosphate Buffer In The Body.
From www.slideserve.com
PPT Fluids and Electrolytes PowerPoint Presentation, free download Phosphate Buffer In The Body Sodium dihydrogen phosphate (na h 2 po 4 na h 2 po 4), which is a weak. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. The phosphate buffer system is a crucial mechanism for regulating the ph of bodily fluids, particularly in the kidneys. Phosphates are found in the blood in two. Phosphate Buffer In The Body.
From www.slideserve.com
PPT Buffer Systems of the Body PowerPoint Presentation, free download Phosphate Buffer In The Body Phosphates are found in the blood in two forms: Sodium dihydrogen phosphate (na h 2 po 4 na h 2 po 4), which is a weak. The concentration of phosphate in the blood is so. A variety of buffering systems exist in the body that helps maintain the ph of the blood and other fluids within a narrow. Sodium dihydrogen. Phosphate Buffer In The Body.
From www.researchgate.net
Diagrammatic representation of phosphate balance in humans. Download Phosphate Buffer In The Body Phosphates are found in the blood in two forms: All of these contain bases that accept hydrogen. Sodium dihydrogen phosphate (na h 2 po 4 na h 2 po 4), which is a weak. Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. Phosphates are found in the blood in two forms: Phosphates are. Phosphate Buffer In The Body.
From www.slideserve.com
PPT Buffer systems PowerPoint Presentation, free download ID9427698 Phosphate Buffer In The Body Sodium dihydrogen phosphate (na h 2 po 4 na h 2 po 4), which is a weak. Phosphates are found in the blood in two forms: Phosphates are found in the blood in two forms: All of these contain bases that accept hydrogen. The phosphate buffer system is not an important blood buffer as its concentration is too low. The. Phosphate Buffer In The Body.
From www.slideserve.com
PPT Buffer Systems of the Body PowerPoint Presentation, free download Phosphate Buffer In The Body Sodium dihydrogen phosphate (na h 2 po 4 na h 2 po 4), which is a weak. The phosphate buffer system is a crucial mechanism for regulating the ph of bodily fluids, particularly in the kidneys. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. All of these contain bases that accept hydrogen.. Phosphate Buffer In The Body.
From www.slideshare.net
Buffer system Phosphate Buffer In The Body Phosphates are found in the blood in two forms: Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. Phosphates are found in the blood in two forms: The phosphate buffer system is a crucial mechanism for regulating the ph of bodily fluids, particularly in the kidneys. Sodium dihydrogen phosphate (na 2 h 2. Phosphate Buffer In The Body.
From www.slideserve.com
PPT Experiment No 3 PowerPoint Presentation, free download ID4828328 Phosphate Buffer In The Body Sodium dihydrogen phosphate (na h 2 po 4 na h 2 po 4), which is a weak. Phosphates are found in the blood in two forms: The phosphate buffer system is not an important blood buffer as its concentration is too low. The concentration of phosphate in the blood is so. The phosphate buffer system is a crucial mechanism for. Phosphate Buffer In The Body.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID Phosphate Buffer In The Body Sodium dihydrogen phosphate (na h 2 po 4 na h 2 po 4), which is a weak. Phosphates are found in the blood in two forms: Phosphates are found in the blood in two forms: Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. The concentration of phosphate in the blood is so.. Phosphate Buffer In The Body.
From www.slideserve.com
PPT Unit Five The Body Fluids and Kidneys PowerPoint Presentation Phosphate Buffer In The Body Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and. A variety of buffering systems exist in the body that helps maintain the ph of the blood and other fluids within a narrow. Sodium dihydrogen phosphate (na h 2 po 4 na h 2 po 4), which is a weak. Phosphates are found in. Phosphate Buffer In The Body.
From animalia-life.club
Phosphate Buffer System Equation Phosphate Buffer In The Body Phosphates are found in the blood in two forms: Sodium dihydrogen phosphate (na h 2 po 4 na h 2 po 4), which is a weak. Phosphates are found in the blood in two forms: All of these contain bases that accept hydrogen. Phosphates are found in the blood in two forms: Sodium dihydrogen phosphate (na 2 h 2 po. Phosphate Buffer In The Body.
From www.zuniv.net
New Human Physiology Ch 17 Phosphate Buffer In The Body Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and sodium monohydrogen phosphate (na. Phosphates are found in the blood in two forms: The concentration of phosphate in the blood is so. The phosphate buffer system is not an important blood buffer as its concentration is too low. All of these contain bases that. Phosphate Buffer In The Body.
From slideplayer.com
Chapter 24 *APR Lecture PowerPoint ppt download Phosphate Buffer In The Body Sodium dihydrogen phosphate (na h 2 po 4 na h 2 po 4), which is a weak. A variety of buffering systems exist in the body that helps maintain the ph of the blood and other fluids within a narrow. Sodium dihydrogen phosphate (na 2 h 2 po 4 −), which is a weak acid, and sodium monohydrogen phosphate (na.. Phosphate Buffer In The Body.