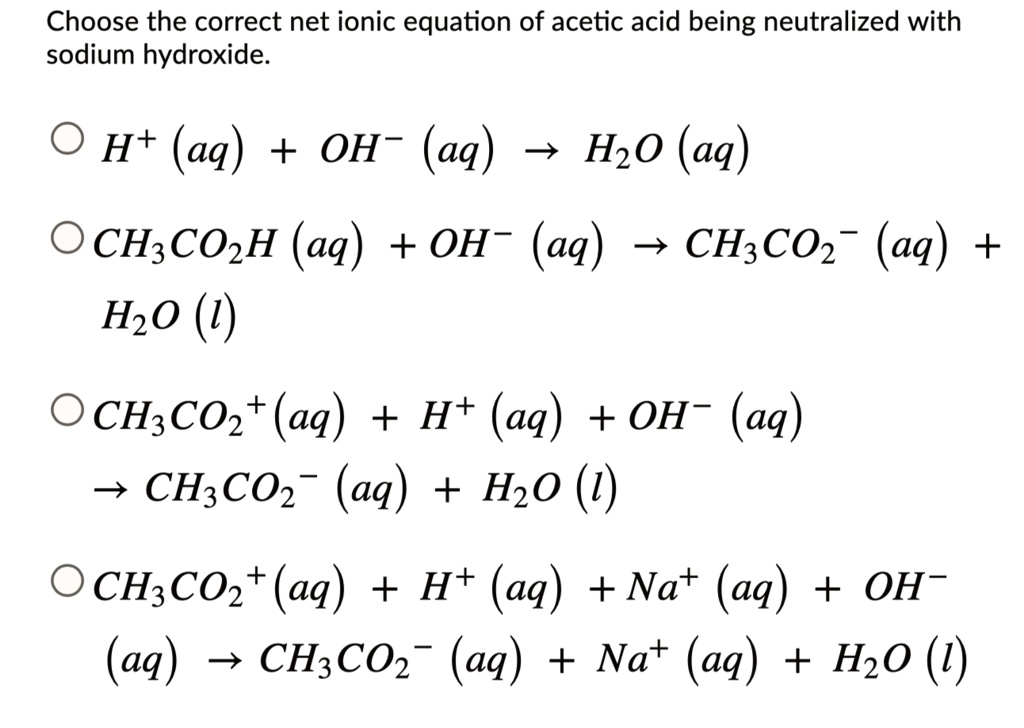
Acetic Acid + Sodium Hydroxide . Initially the ph is due to pure acetic acid. when acetic acid ch 3 cooh reacts with sodium hydroxide naoh, the formation of s odium acetate ch 3 coona, and water h 2. — there are three main steps for writing the net ionic equation for acetic. — acetic acid (hc2h3o2) is the active ingredient in vinegar and is responsible for its sour taste. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. take, for example, the reaction being investigated today, that between solutions of acetic acid and sodium hydroxide, naoh. — the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. — acetic acid, ch3cooh, will react with sodium hydroxide, naoh, to produce sodium acetate, ch3coona, and. — in this lab, you will perform a titration using sodium hydroxide and acetic acid (in vinegar). Write the balanced neutralization reaction.
from www.numerade.com
Initially the ph is due to pure acetic acid. when acetic acid ch 3 cooh reacts with sodium hydroxide naoh, the formation of s odium acetate ch 3 coona, and water h 2. take, for example, the reaction being investigated today, that between solutions of acetic acid and sodium hydroxide, naoh. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. — the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. — there are three main steps for writing the net ionic equation for acetic. — acetic acid (hc2h3o2) is the active ingredient in vinegar and is responsible for its sour taste. — in this lab, you will perform a titration using sodium hydroxide and acetic acid (in vinegar). — acetic acid, ch3cooh, will react with sodium hydroxide, naoh, to produce sodium acetate, ch3coona, and. Write the balanced neutralization reaction.
SOLVED Choose the correct net ionic equation of acetic acid being
Acetic Acid + Sodium Hydroxide — there are three main steps for writing the net ionic equation for acetic. Write the balanced neutralization reaction. when acetic acid ch 3 cooh reacts with sodium hydroxide naoh, the formation of s odium acetate ch 3 coona, and water h 2. — acetic acid, ch3cooh, will react with sodium hydroxide, naoh, to produce sodium acetate, ch3coona, and. — acetic acid (hc2h3o2) is the active ingredient in vinegar and is responsible for its sour taste. — in this lab, you will perform a titration using sodium hydroxide and acetic acid (in vinegar). titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. take, for example, the reaction being investigated today, that between solutions of acetic acid and sodium hydroxide, naoh. Initially the ph is due to pure acetic acid. — there are three main steps for writing the net ionic equation for acetic. — the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion.
From www.researchgate.net
Dependences of the densities of the solutions of acetic acid (1 Acetic Acid + Sodium Hydroxide when acetic acid ch 3 cooh reacts with sodium hydroxide naoh, the formation of s odium acetate ch 3 coona, and water h 2. — there are three main steps for writing the net ionic equation for acetic. — in this lab, you will perform a titration using sodium hydroxide and acetic acid (in vinegar). take,. Acetic Acid + Sodium Hydroxide.
From www.numerade.com
SOLVEDMixing together solutions of acetic acid and sodium hydroxide Acetic Acid + Sodium Hydroxide take, for example, the reaction being investigated today, that between solutions of acetic acid and sodium hydroxide, naoh. — the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. — in this lab, you will perform a titration using sodium hydroxide and acetic. Acetic Acid + Sodium Hydroxide.
From thisnutrition.com
Acetic Acid And Sodium Hydroxide This Nutrition Acetic Acid + Sodium Hydroxide — acetic acid (hc2h3o2) is the active ingredient in vinegar and is responsible for its sour taste. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. when acetic acid ch 3 cooh reacts with sodium hydroxide naoh, the formation of s odium acetate ch 3 coona, and water h 2. take, for example, the. Acetic Acid + Sodium Hydroxide.
From www.youtube.com
Write the balanced chemical equation for the reaction of sodium Acetic Acid + Sodium Hydroxide Write the balanced neutralization reaction. when acetic acid ch 3 cooh reacts with sodium hydroxide naoh, the formation of s odium acetate ch 3 coona, and water h 2. — the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. take, for example,. Acetic Acid + Sodium Hydroxide.
From www.youtube.com
Acetic acid (ethanoic acid) and Sodium hydroxide reaction CH3COOH Acetic Acid + Sodium Hydroxide when acetic acid ch 3 cooh reacts with sodium hydroxide naoh, the formation of s odium acetate ch 3 coona, and water h 2. — acetic acid (hc2h3o2) is the active ingredient in vinegar and is responsible for its sour taste. — acetic acid, ch3cooh, will react with sodium hydroxide, naoh, to produce sodium acetate, ch3coona, and.. Acetic Acid + Sodium Hydroxide.
From www.chegg.com
Solved Acetic acid reacts with sodium hydroxide according Acetic Acid + Sodium Hydroxide — in this lab, you will perform a titration using sodium hydroxide and acetic acid (in vinegar). — acetic acid, ch3cooh, will react with sodium hydroxide, naoh, to produce sodium acetate, ch3coona, and. — there are three main steps for writing the net ionic equation for acetic. — the titration of a weak acid with a. Acetic Acid + Sodium Hydroxide.
From brainly.in
What type of salt is obtained when sodium hydroxide reacts with acetic Acetic Acid + Sodium Hydroxide take, for example, the reaction being investigated today, that between solutions of acetic acid and sodium hydroxide, naoh. Write the balanced neutralization reaction. Initially the ph is due to pure acetic acid. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. — there are three main steps for writing the net ionic equation for acetic.. Acetic Acid + Sodium Hydroxide.
From chempedia.info
Sodium hydroxide titration with acetic acid Big Chemical Encyclopedia Acetic Acid + Sodium Hydroxide — acetic acid (hc2h3o2) is the active ingredient in vinegar and is responsible for its sour taste. — acetic acid, ch3cooh, will react with sodium hydroxide, naoh, to produce sodium acetate, ch3coona, and. when acetic acid ch 3 cooh reacts with sodium hydroxide naoh, the formation of s odium acetate ch 3 coona, and water h 2.. Acetic Acid + Sodium Hydroxide.
From readingandwritingprojectcom.web.fc2.com
acetic acid reacts with sodium hydroxide Acetic Acid + Sodium Hydroxide titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. when acetic acid ch 3 cooh reacts with sodium hydroxide naoh, the formation of s odium acetate ch 3 coona, and water h 2. — there are three main steps for writing the net ionic equation for acetic. Initially the ph is due to pure acetic. Acetic Acid + Sodium Hydroxide.
From lessonmagicgast.z22.web.core.windows.net
The Equation Shows A Neutralization Reaction Acetic Acid + Sodium Hydroxide Write the balanced neutralization reaction. when acetic acid ch 3 cooh reacts with sodium hydroxide naoh, the formation of s odium acetate ch 3 coona, and water h 2. take, for example, the reaction being investigated today, that between solutions of acetic acid and sodium hydroxide, naoh. — acetic acid (hc2h3o2) is the active ingredient in vinegar. Acetic Acid + Sodium Hydroxide.
From fr.slideserve.com
PPT Common Types of Reactions PowerPoint Presentation, free download Acetic Acid + Sodium Hydroxide — in this lab, you will perform a titration using sodium hydroxide and acetic acid (in vinegar). — there are three main steps for writing the net ionic equation for acetic. take, for example, the reaction being investigated today, that between solutions of acetic acid and sodium hydroxide, naoh. Write the balanced neutralization reaction. — acetic. Acetic Acid + Sodium Hydroxide.
From www.toppr.com
The enthalpy of neutralization of acetic acid and sodium hydroxide is Acetic Acid + Sodium Hydroxide — there are three main steps for writing the net ionic equation for acetic. — the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. — acetic acid (hc2h3o2) is the active ingredient in vinegar and is responsible for its sour taste. . Acetic Acid + Sodium Hydroxide.
From clarissa-bloggay.blogspot.com
Balanced Equation for the Neutralization of Acetic Acid Acetic Acid + Sodium Hydroxide — there are three main steps for writing the net ionic equation for acetic. — the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Write the balanced neutralization reaction. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. Initially the. Acetic Acid + Sodium Hydroxide.
From www.numerade.com
SOLVED Sodium hydroxide (base) and acetic acid react in a 11 molar Acetic Acid + Sodium Hydroxide — acetic acid (hc2h3o2) is the active ingredient in vinegar and is responsible for its sour taste. — in this lab, you will perform a titration using sodium hydroxide and acetic acid (in vinegar). — there are three main steps for writing the net ionic equation for acetic. Initially the ph is due to pure acetic acid.. Acetic Acid + Sodium Hydroxide.
From clarissa-bloggay.blogspot.com
Balanced Equation for the Neutralization of Acetic Acid Acetic Acid + Sodium Hydroxide titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. — acetic acid, ch3cooh, will react with sodium hydroxide, naoh, to produce sodium acetate, ch3coona, and. — there are three main steps for writing the net ionic equation for acetic. — acetic acid (hc2h3o2) is the active ingredient in vinegar and is responsible for its. Acetic Acid + Sodium Hydroxide.
From www.numerade.com
SOLVED 5 Using the determined equivalence point from question 2 and Acetic Acid + Sodium Hydroxide when acetic acid ch 3 cooh reacts with sodium hydroxide naoh, the formation of s odium acetate ch 3 coona, and water h 2. — in this lab, you will perform a titration using sodium hydroxide and acetic acid (in vinegar). — acetic acid (hc2h3o2) is the active ingredient in vinegar and is responsible for its sour. Acetic Acid + Sodium Hydroxide.
From readingandwritingprojectcom.web.fc2.com
acetic acid reacts with sodium hydroxide Acetic Acid + Sodium Hydroxide — the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. take, for example, the reaction being investigated today, that between solutions of acetic acid and sodium hydroxide, naoh. Initially the ph is due to pure acetic acid. Write the balanced neutralization reaction. . Acetic Acid + Sodium Hydroxide.
From www.slideserve.com
PPT Reaction of Acetic Acid with Hydroxide Ion PowerPoint Acetic Acid + Sodium Hydroxide — acetic acid, ch3cooh, will react with sodium hydroxide, naoh, to produce sodium acetate, ch3coona, and. — the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. take, for example, the reaction being investigated today, that between solutions of acetic acid and sodium. Acetic Acid + Sodium Hydroxide.
From www.numerade.com
SOLVED Write equations for the following reactions, representing the Acetic Acid + Sodium Hydroxide — there are three main steps for writing the net ionic equation for acetic. take, for example, the reaction being investigated today, that between solutions of acetic acid and sodium hydroxide, naoh. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. — the titration of a weak acid with a strong base involves the. Acetic Acid + Sodium Hydroxide.
From uhighlsu.web.fc2.com
sodium hydroxide + acetic acid Acetic Acid + Sodium Hydroxide — the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. Write the balanced neutralization reaction. take, for example, the reaction being investigated today, that between solutions of acetic acid and sodium hydroxide, naoh. titration of acetic acid with sodium hydroxide (analagous to. Acetic Acid + Sodium Hydroxide.
From clarissa-bloggay.blogspot.com
Balanced Equation for the Neutralization of Acetic Acid Acetic Acid + Sodium Hydroxide Write the balanced neutralization reaction. when acetic acid ch 3 cooh reacts with sodium hydroxide naoh, the formation of s odium acetate ch 3 coona, and water h 2. Initially the ph is due to pure acetic acid. — acetic acid (hc2h3o2) is the active ingredient in vinegar and is responsible for its sour taste. — there. Acetic Acid + Sodium Hydroxide.
From readingandwritingprojectcom.web.fc2.com
acetic acid reacts with sodium hydroxide Acetic Acid + Sodium Hydroxide Write the balanced neutralization reaction. — there are three main steps for writing the net ionic equation for acetic. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. when acetic acid ch 3 cooh reacts with sodium hydroxide naoh, the formation of s odium acetate ch 3 coona, and water h 2. — acetic. Acetic Acid + Sodium Hydroxide.
From www.numerade.com
SOLVED 3. White the reaction between acetic acid and sodium hydroxide. Acetic Acid + Sodium Hydroxide — acetic acid, ch3cooh, will react with sodium hydroxide, naoh, to produce sodium acetate, ch3coona, and. — acetic acid (hc2h3o2) is the active ingredient in vinegar and is responsible for its sour taste. — in this lab, you will perform a titration using sodium hydroxide and acetic acid (in vinegar). — the titration of a weak. Acetic Acid + Sodium Hydroxide.
From www.youtube.com
ACETIC ACID TITRATION BY SODIUM HYDROXIDE YouTube Acetic Acid + Sodium Hydroxide when acetic acid ch 3 cooh reacts with sodium hydroxide naoh, the formation of s odium acetate ch 3 coona, and water h 2. — acetic acid (hc2h3o2) is the active ingredient in vinegar and is responsible for its sour taste. — there are three main steps for writing the net ionic equation for acetic. —. Acetic Acid + Sodium Hydroxide.
From www.chegg.com
Solved What is the net ionic equation for the reaction of Acetic Acid + Sodium Hydroxide Initially the ph is due to pure acetic acid. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. when acetic acid ch 3 cooh reacts with sodium hydroxide naoh, the formation of s odium acetate ch 3 coona, and water h 2. Write the balanced neutralization reaction. — in this lab, you will perform a. Acetic Acid + Sodium Hydroxide.
From mungfali.com
Titration Curve Of Acetic Acid Acetic Acid + Sodium Hydroxide — in this lab, you will perform a titration using sodium hydroxide and acetic acid (in vinegar). take, for example, the reaction being investigated today, that between solutions of acetic acid and sodium hydroxide, naoh. — acetic acid, ch3cooh, will react with sodium hydroxide, naoh, to produce sodium acetate, ch3coona, and. — acetic acid (hc2h3o2) is. Acetic Acid + Sodium Hydroxide.
From www.youtube.com
How to Write the Net Ionic Equation for Acetic acid + Sodium hydroxide Acetic Acid + Sodium Hydroxide take, for example, the reaction being investigated today, that between solutions of acetic acid and sodium hydroxide, naoh. Initially the ph is due to pure acetic acid. — there are three main steps for writing the net ionic equation for acetic. — the titration of a weak acid with a strong base involves the direct transfer of. Acetic Acid + Sodium Hydroxide.
From www.numerade.com
SOLVED For the reaction of acetic acid, CH3COOH, with sodium hydroxide Acetic Acid + Sodium Hydroxide — the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. — acetic acid, ch3cooh, will react with sodium hydroxide, naoh, to produce sodium acetate, ch3coona, and. — there are three main steps for writing the net ionic equation for acetic. when. Acetic Acid + Sodium Hydroxide.
From www.youtube.com
Sodium hydroxide solution is treated with acetic acid to formsodium Acetic Acid + Sodium Hydroxide take, for example, the reaction being investigated today, that between solutions of acetic acid and sodium hydroxide, naoh. Write the balanced neutralization reaction. — in this lab, you will perform a titration using sodium hydroxide and acetic acid (in vinegar). Initially the ph is due to pure acetic acid. — the titration of a weak acid with. Acetic Acid + Sodium Hydroxide.
From mavink.com
Acetic Acid And Sodium Hydroxide Reaction Acetic Acid + Sodium Hydroxide take, for example, the reaction being investigated today, that between solutions of acetic acid and sodium hydroxide, naoh. when acetic acid ch 3 cooh reacts with sodium hydroxide naoh, the formation of s odium acetate ch 3 coona, and water h 2. — there are three main steps for writing the net ionic equation for acetic. . Acetic Acid + Sodium Hydroxide.
From www.youtube.com
chemical equation of reaction of ethanoic acid/acetic acid with sodium Acetic Acid + Sodium Hydroxide titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. Write the balanced neutralization reaction. Initially the ph is due to pure acetic acid. when acetic acid ch 3 cooh reacts with sodium hydroxide naoh, the formation of s odium acetate ch 3 coona, and water h 2. — acetic acid (hc2h3o2) is the active ingredient. Acetic Acid + Sodium Hydroxide.
From www.numerade.com
SOLVED Choose the correct net ionic equation of acetic acid being Acetic Acid + Sodium Hydroxide when acetic acid ch 3 cooh reacts with sodium hydroxide naoh, the formation of s odium acetate ch 3 coona, and water h 2. — in this lab, you will perform a titration using sodium hydroxide and acetic acid (in vinegar). — acetic acid (hc2h3o2) is the active ingredient in vinegar and is responsible for its sour. Acetic Acid + Sodium Hydroxide.
From www.youtube.com
Acetic acid and sodium hydroxide YouTube Acetic Acid + Sodium Hydroxide — in this lab, you will perform a titration using sodium hydroxide and acetic acid (in vinegar). — the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. take, for example, the reaction being investigated today, that between solutions of acetic acid and. Acetic Acid + Sodium Hydroxide.
From readingandwritingprojectcom.web.fc2.com
acetic acid reacts with sodium hydroxide Acetic Acid + Sodium Hydroxide — in this lab, you will perform a titration using sodium hydroxide and acetic acid (in vinegar). take, for example, the reaction being investigated today, that between solutions of acetic acid and sodium hydroxide, naoh. when acetic acid ch 3 cooh reacts with sodium hydroxide naoh, the formation of s odium acetate ch 3 coona, and water. Acetic Acid + Sodium Hydroxide.
From www.chegg.com
Solved Acetic acid reacts with sodium hydroxide according Acetic Acid + Sodium Hydroxide — there are three main steps for writing the net ionic equation for acetic. — acetic acid, ch3cooh, will react with sodium hydroxide, naoh, to produce sodium acetate, ch3coona, and. Initially the ph is due to pure acetic acid. — in this lab, you will perform a titration using sodium hydroxide and acetic acid (in vinegar). . Acetic Acid + Sodium Hydroxide.