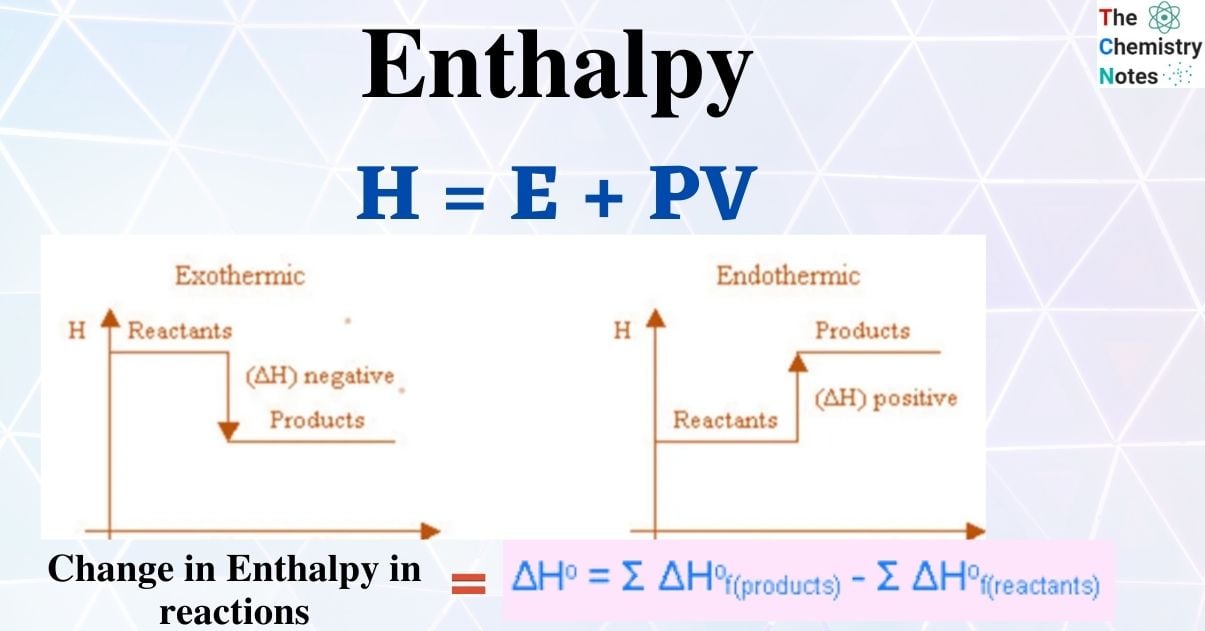
Standard Enthalpy Of Formation Equal To Zero . Oxygen (the element) at standard state is o 2. To determine which form is zero, the more stable form of carbon is chosen. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. This is also the form with the lowest enthalpy, so graphite has a standard. The standard enthalpy of formation for an element in its standard state is zero!!!! By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is. The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its graphite form. The standard enthalpy of formation δh∘f δ h f ° of pure elements is zero by definition. It is true that enthalpy. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. The standard enthalpy of formation of any element in its standard state is zero by definition. Elements in their standard state are not formed, they just are. Why is that a convention?
from scienceinfo.com
By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. This is also the form with the lowest enthalpy, so graphite has a standard. Oxygen (the element) at standard state is o 2. The standard enthalpy of formation of any element in its standard state is zero by definition. The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its graphite form. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is. Elements in their standard state are not formed, they just are. The standard enthalpy of formation δh∘f δ h f ° of pure elements is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for.
Enthalpy Introduction, Calculation, Enthalpy change, Importance
Standard Enthalpy Of Formation Equal To Zero The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation for an element in its standard state is zero!!!! The standard enthalpy of formation δh∘f δ h f ° of pure elements is zero by definition. It is true that enthalpy. Why is that a convention? For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. Oxygen (the element) at standard state is o 2. The standard enthalpy of formation of any element in its standard state is zero by definition. Elements in their standard state are not formed, they just are. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. This is also the form with the lowest enthalpy, so graphite has a standard. To determine which form is zero, the more stable form of carbon is chosen. The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its graphite form.
From studyafrikander.z13.web.core.windows.net
Calculate Standard Enthalpy Of Reaction Standard Enthalpy Of Formation Equal To Zero For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is. This is also the form with the lowest enthalpy, so graphite has a standard. To determine which form is zero, the more stable form of carbon is chosen. Oxygen (the element) at standard state is o 2.. Standard Enthalpy Of Formation Equal To Zero.
From slideplayer.com
Standard Enthalpies of Formation ppt download Standard Enthalpy Of Formation Equal To Zero Why is that a convention? This is also the form with the lowest enthalpy, so graphite has a standard. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat. Standard Enthalpy Of Formation Equal To Zero.
From www.chegg.com
Solved Which of the following has a standard enthalpy of Standard Enthalpy Of Formation Equal To Zero 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Oxygen (the element) at standard state is o 2. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. The. Standard Enthalpy Of Formation Equal To Zero.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation Equal To Zero Oxygen (the element) at standard state is o 2. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is. By definition, the standard enthalpy. Standard Enthalpy Of Formation Equal To Zero.
From www.slideserve.com
PPT Energy changes PowerPoint Presentation, free download ID3003657 Standard Enthalpy Of Formation Equal To Zero The standard enthalpy of formation for an element in its standard state is zero!!!! By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. It is true that enthalpy. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat. Standard Enthalpy Of Formation Equal To Zero.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation Equal To Zero The standard enthalpy of formation of any element in its standard state is zero by definition. Why is that a convention? The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its graphite form. By definition, the standard enthalpy of formation of an element in its most stable form is. Standard Enthalpy Of Formation Equal To Zero.
From www.chem.fsu.edu
CHM1045 Enthalpy Lecture Standard Enthalpy Of Formation Equal To Zero For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is. The standard enthalpy of formation of any element in its standard state is zero by definition. It is true that enthalpy. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation. Standard Enthalpy Of Formation Equal To Zero.
From slideplayer.com
Standard Enthalpy of Formation EQ Why does the Hfº for a free element Standard Enthalpy Of Formation Equal To Zero It is true that enthalpy. The standard enthalpy of formation for an element in its standard state is zero!!!! The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its graphite form. The standard enthalpy of formation δh∘f δ h f ° of pure elements is zero by definition. This. Standard Enthalpy Of Formation Equal To Zero.
From www.chegg.com
Solved 3. Which of the following does not have a standard Standard Enthalpy Of Formation Equal To Zero 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is. Oxygen (the element) at standard state is o 2. The standard enthalpy of formation. Standard Enthalpy Of Formation Equal To Zero.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Of Formation Equal To Zero Oxygen (the element) at standard state is o 2. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone. Standard Enthalpy Of Formation Equal To Zero.
From www.chegg.com
Solved Which substances below have a standard enthalpy of Standard Enthalpy Of Formation Equal To Zero By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. For example, although oxygen can exist. Standard Enthalpy Of Formation Equal To Zero.
From schoolworkhelper.net
Standard Enthalpies of Formation Online Homework Help SchoolWorkHelper Standard Enthalpy Of Formation Equal To Zero The standard enthalpy of formation of any element in its standard state is zero by definition. Why is that a convention? It is true that enthalpy. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. Oxygen (the element) at standard state is. Standard Enthalpy Of Formation Equal To Zero.
From www.numerade.com
SOLVED Calculate the standard enthalpy of formation of Cl(aq) given Standard Enthalpy Of Formation Equal To Zero For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation δh∘f δ h f ° of pure elements is zero by definition. It is true that. Standard Enthalpy Of Formation Equal To Zero.
From slideplayer.com
Standard Enthalpy of Formation EQ Why does the Hfº for a free element Standard Enthalpy Of Formation Equal To Zero Why is that a convention? By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its graphite form. 193 rows in chemistry and. Standard Enthalpy Of Formation Equal To Zero.
From www.numerade.com
SOLVED Question 13 pts Which of the following does not have a standard Standard Enthalpy Of Formation Equal To Zero The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its graphite form. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is. Why is that a convention? To determine which form is zero, the more stable. Standard Enthalpy Of Formation Equal To Zero.
From www.chegg.com
Solved Which of the following substances has a standard Standard Enthalpy Of Formation Equal To Zero By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. Elements in their standard state are not formed, they just are. The standard enthalpy of formation δh∘f δ h f ° of pure elements is zero by definition. It is true that enthalpy.. Standard Enthalpy Of Formation Equal To Zero.
From www.slideserve.com
PPT Unit 7 Review Game Board PowerPoint Presentation, free download Standard Enthalpy Of Formation Equal To Zero The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is. This is also the form with the lowest enthalpy, so graphite has a standard. To determine which form is zero, the. Standard Enthalpy Of Formation Equal To Zero.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation Equal To Zero To determine which form is zero, the more stable form of carbon is chosen. The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its graphite form. Elements in their standard state are not formed, they just are. Why is that a convention? The standard enthalpy of formation of any. Standard Enthalpy Of Formation Equal To Zero.
From www.numerade.com
Select which of the following standard enthalpy of formation values is Standard Enthalpy Of Formation Equal To Zero The standard enthalpy of formation δh∘f δ h f ° of pure elements is zero by definition. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. This is also the form with the lowest enthalpy, so graphite has a standard. 193 rows. Standard Enthalpy Of Formation Equal To Zero.
From scienceinfo.com
Enthalpy Introduction, Calculation, Enthalpy change, Importance Standard Enthalpy Of Formation Equal To Zero It is true that enthalpy. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Elements in their standard state are not formed, they just are. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. Standard Enthalpy Of Formation Equal To Zero.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Of Formation Equal To Zero Why is that a convention? It is true that enthalpy. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. To determine which form is zero, the more stable form of carbon is chosen. By definition, the standard enthalpy of formation of an element in its most. Standard Enthalpy Of Formation Equal To Zero.
From cewuaeqb.blob.core.windows.net
How Do You Work Out Standard Enthalpy Of Formation at Connie Stroud blog Standard Enthalpy Of Formation Equal To Zero The standard enthalpy of formation of any element in its standard state is zero by definition. It is true that enthalpy. Why is that a convention? The standard enthalpy of formation for an element in its standard state is zero!!!! By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under. Standard Enthalpy Of Formation Equal To Zero.
From www.doubtnut.com
Which of these species has a standard enthalpy of formation equal to z Standard Enthalpy Of Formation Equal To Zero This is also the form with the lowest enthalpy, so graphite has a standard. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. The standard enthalpy of formation of any element in its standard state is zero by definition. Elements in their. Standard Enthalpy Of Formation Equal To Zero.
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation Standard Enthalpy Of Formation Equal To Zero The standard enthalpy of formation for an element in its standard state is zero!!!! Oxygen (the element) at standard state is o 2. It is true that enthalpy. Elements in their standard state are not formed, they just are. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o. Standard Enthalpy Of Formation Equal To Zero.
From www.numerade.com
SOLVED Mark each substance which has an enthalpy of formation equal to Standard Enthalpy Of Formation Equal To Zero By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. Elements in their standard state are not formed, they just are. The standard enthalpy of formation δh∘f δ h f ° of pure elements is zero by definition. This is also the form. Standard Enthalpy Of Formation Equal To Zero.
From www.thoughtco.com
Why the Standard Enthalpy of Formation of O2 Equals Zero Standard Enthalpy Of Formation Equal To Zero The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation δh∘f δ h f ° of pure elements is zero by definition. This is also the form with the lowest enthalpy, so graphite has a standard. To determine which form is zero, the more stable form of carbon is. Standard Enthalpy Of Formation Equal To Zero.
From www.slideserve.com
PPT Standard Enthalpy (Ch_6.6) PowerPoint Presentation, free download Standard Enthalpy Of Formation Equal To Zero This is also the form with the lowest enthalpy, so graphite has a standard. It is true that enthalpy. The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The same. Standard Enthalpy Of Formation Equal To Zero.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation Equal To Zero The standard enthalpy of formation for an element in its standard state is zero!!!! 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation δh∘f δ h f ° of pure elements is zero by definition. By definition, the standard enthalpy of. Standard Enthalpy Of Formation Equal To Zero.
From www.slideshare.net
Standard enthalpy of formation Standard Enthalpy Of Formation Equal To Zero The standard enthalpy of formation δh∘f δ h f ° of pure elements is zero by definition. The standard enthalpy of formation of any element in its standard state is zero by definition. The same is true other other gaseous elements, such as hydrogen and nitrogen, and solid elements, such as carbon in its graphite form. Why is that a. Standard Enthalpy Of Formation Equal To Zero.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Equal To Zero For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is. Elements in their standard state are not formed, they just are. To determine which form is zero, the more stable form of carbon is chosen. Why is that a convention? It is true that enthalpy. The standard. Standard Enthalpy Of Formation Equal To Zero.
From www.chegg.com
Solved For which of the following substances is the standard Standard Enthalpy Of Formation Equal To Zero The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard. Standard Enthalpy Of Formation Equal To Zero.
From www.youtube.com
Standard Enthalpy of Formation and Formation Reactions OpenStax Standard Enthalpy Of Formation Equal To Zero Why is that a convention? For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen (o 2 ), o 2 is. Oxygen (the element) at standard state is o 2. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which. Standard Enthalpy Of Formation Equal To Zero.
From www.youtube.com
15.1.1 Define Standard State, Enthalpy change of formation and Standard Enthalpy Of Formation Equal To Zero 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Why is that a convention? By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. Elements in their standard state. Standard Enthalpy Of Formation Equal To Zero.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Formation Equal To Zero It is true that enthalpy. Elements in their standard state are not formed, they just are. By definition, the standard enthalpy of formation of an element in its most stable form is equal to zero under standard conditions, which is 1 atm for. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and molecular oxygen. Standard Enthalpy Of Formation Equal To Zero.
From www.slideserve.com
PPT Chapter 15 Standard enthalpy change of a reaction PowerPoint Standard Enthalpy Of Formation Equal To Zero It is true that enthalpy. The standard enthalpy of formation δh∘f δ h f ° of pure elements is zero by definition. To determine which form is zero, the more stable form of carbon is chosen. This is also the form with the lowest enthalpy, so graphite has a standard. Elements in their standard state are not formed, they just. Standard Enthalpy Of Formation Equal To Zero.