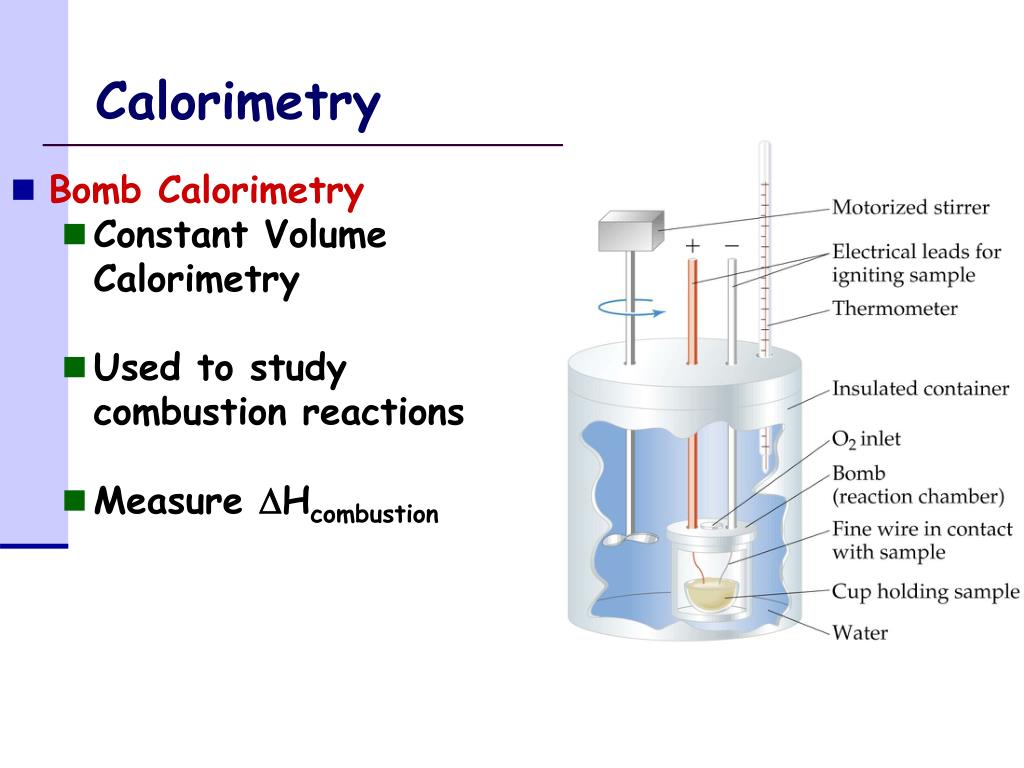
What Are Calorimeters . It uses devices called calorimeters, which measure the change in temperature when a chemical reaction is carried out. Chemists use calorimetry to determine the amount of heat. The magnitude of the temperature change depends on the amount. We will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at different temperatures, and that released or absorbed by. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. It mainly consists of a metallic vessel made of materials which are good conductors of electricity such as copper and aluminium etc. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. We will consider two types of calorimeters, ideal calorimeters, where the calorimeter does not absorb heat itself, and real calorimeters, where the calorimeter absorbs heat. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter is a device used for heat measurements necessary for calorimetry.
from www.slideserve.com
It uses devices called calorimeters, which measure the change in temperature when a chemical reaction is carried out. The magnitude of the temperature change depends on the amount. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. We will consider two types of calorimeters, ideal calorimeters, where the calorimeter does not absorb heat itself, and real calorimeters, where the calorimeter absorbs heat. We will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at different temperatures, and that released or absorbed by. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. A calorimeter is a device used for heat measurements necessary for calorimetry. Chemists use calorimetry to determine the amount of heat. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity.
PPT Calorimetry PowerPoint Presentation, free download ID6133898
What Are Calorimeters We will consider two types of calorimeters, ideal calorimeters, where the calorimeter does not absorb heat itself, and real calorimeters, where the calorimeter absorbs heat. It mainly consists of a metallic vessel made of materials which are good conductors of electricity such as copper and aluminium etc. A calorimeter is a device used for heat measurements necessary for calorimetry. We will consider two types of calorimeters, ideal calorimeters, where the calorimeter does not absorb heat itself, and real calorimeters, where the calorimeter absorbs heat. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. It uses devices called calorimeters, which measure the change in temperature when a chemical reaction is carried out. The magnitude of the temperature change depends on the amount. We will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at different temperatures, and that released or absorbed by. Chemists use calorimetry to determine the amount of heat. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals What Are Calorimeters The magnitude of the temperature change depends on the amount. We will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at different temperatures, and that released or absorbed by. A calorimeter is a device used for heat measurements necessary for calorimetry. Calorimetry is a field of thermochemistry that measures the amount of heat involved. What Are Calorimeters.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 What Are Calorimeters To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. The magnitude of the temperature change depends on the amount. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. We will apply calorimetry to two different thermodynamic processes, that of transference of heat across. What Are Calorimeters.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry What Are Calorimeters We will consider two types of calorimeters, ideal calorimeters, where the calorimeter does not absorb heat itself, and real calorimeters, where the calorimeter absorbs heat. Chemists use calorimetry to determine the amount of heat. The magnitude of the temperature change depends on the amount. It uses devices called calorimeters, which measure the change in temperature when a chemical reaction is. What Are Calorimeters.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals What Are Calorimeters To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. A calorimeter is a device used for heat measurements necessary for calorimetry. It uses devices called calorimeters, which measure the change in temperature when a chemical reaction is carried out. Chemists use calorimetry to determine the amount of heat. We will apply calorimetry to two. What Are Calorimeters.
From glossary.periodni.com
Bomb calorimeter Chemistry Dictionary & Glossary What Are Calorimeters It mainly consists of a metallic vessel made of materials which are good conductors of electricity such as copper and aluminium etc. We will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at different temperatures, and that released or absorbed by. Calorimetry is a field of thermochemistry that measures the amount of heat involved. What Are Calorimeters.
From studylib.net
Calorimetry What Are Calorimeters A calorimeter is a device used for heat measurements necessary for calorimetry. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. We will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at different temperatures, and that released or absorbed by. Calorimetry is a branch. What Are Calorimeters.
From chemlab.truman.edu
Parr 1341 Bomb Calorimeter Chem Lab What Are Calorimeters To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. It mainly consists of a metallic vessel made of materials which are good conductors of electricity such as copper and aluminium etc. A calorimeter is a device used for heat measurements necessary for calorimetry. We will apply calorimetry to two different thermodynamic processes, that of. What Are Calorimeters.
From eduinput.com
10 Examples of Plasma in Physics What Are Calorimeters Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. We will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at different temperatures, and that released or absorbed by. Calorimetry is a field of thermochemistry that measures the amount of. What Are Calorimeters.
From nerdtechy.com
Ultimate Guide to the Best Calorimeters for 20202021 What Are Calorimeters The magnitude of the temperature change depends on the amount. A calorimeter is a device used for heat measurements necessary for calorimetry. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Chemists use calorimetry. What Are Calorimeters.
From www.laboratory-equipment.com
Laboratory Calorimeters from IKA What Are Calorimeters We will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at different temperatures, and that released or absorbed by. We will consider two types of calorimeters, ideal calorimeters, where the calorimeter does not absorb heat itself, and real calorimeters, where the calorimeter absorbs heat. Chemists use calorimetry to determine the amount of heat. Calorimetry. What Are Calorimeters.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 What Are Calorimeters Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. We will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at. What Are Calorimeters.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec What Are Calorimeters Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Chemists use calorimetry to determine the amount of heat. It mainly consists of a metallic vessel made of materials which are good conductors of electricity such as copper and aluminium etc. We will consider two types of calorimeters, ideal calorimeters, where. What Are Calorimeters.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation What Are Calorimeters We will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at different temperatures, and that released or absorbed by. It uses devices called calorimeters, which measure the change in temperature when a chemical reaction is carried out. The magnitude of the temperature change depends on the amount. Calorimeter, device for measuring the heat developed. What Are Calorimeters.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson What Are Calorimeters Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. It mainly consists of a metallic vessel made of materials which are good conductors of electricity such as copper and aluminium. What Are Calorimeters.
From faqguide.co
What does a calorimeter do? Explained by FAQGuide What Are Calorimeters The magnitude of the temperature change depends on the amount. It uses devices called calorimeters, which measure the change in temperature when a chemical reaction is carried out. Chemists use calorimetry to determine the amount of heat. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. To determine the enthalpy,. What Are Calorimeters.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle What Are Calorimeters Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. We will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects. What Are Calorimeters.
From www.pinterest.com
Calorimetry Bomb Calorimeter Experiment Science fair, Homeschool and What Are Calorimeters Chemists use calorimetry to determine the amount of heat. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. We will consider two types of calorimeters, ideal calorimeters, where the calorimeter does not absorb heat itself, and real calorimeters, where the calorimeter absorbs heat. It mainly consists of a metallic. What Are Calorimeters.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 What Are Calorimeters The magnitude of the temperature change depends on the amount. It mainly consists of a metallic vessel made of materials which are good conductors of electricity such as copper and aluminium etc. We will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at different temperatures, and that released or absorbed by. Chemists use calorimetry. What Are Calorimeters.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica What Are Calorimeters We will consider two types of calorimeters, ideal calorimeters, where the calorimeter does not absorb heat itself, and real calorimeters, where the calorimeter absorbs heat. The magnitude of the temperature change depends on the amount. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. It mainly consists of a metallic vessel made of materials. What Are Calorimeters.
From pressbooks.calstate.edu
3.1 Calorimetry Nutrition and Physical Fitness What Are Calorimeters Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter is a device used for heat measurements necessary for calorimetry. It mainly consists of a metallic vessel made of materials which are good conductors of electricity such as copper and aluminium etc. To determine the enthalpy, stability, heat capacity,. What Are Calorimeters.
From klayjstec.blob.core.windows.net
How Calorimeter Function at Geneva Katzman blog What Are Calorimeters It uses devices called calorimeters, which measure the change in temperature when a chemical reaction is carried out. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. It mainly consists of a metallic vessel made of materials which are good conductors of electricity such as copper and aluminium etc. A. What Are Calorimeters.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types What Are Calorimeters Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. We will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at different temperatures, and that. What Are Calorimeters.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry What Are Calorimeters The magnitude of the temperature change depends on the amount. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. It uses devices called calorimeters, which measure the change in temperature when a chemical reaction is. What Are Calorimeters.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas What Are Calorimeters It mainly consists of a metallic vessel made of materials which are good conductors of electricity such as copper and aluminium etc. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. We will consider two types of calorimeters, ideal calorimeters, where the calorimeter does not absorb heat itself, and real. What Are Calorimeters.
From www.youtube.com
050 Calorimetry YouTube What Are Calorimeters Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is a device used for heat measurements necessary for calorimetry. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. It uses devices called calorimeters, which measure the change in temperature when a. What Are Calorimeters.
From www.slideserve.com
PPT An introduction to calorimeters for particle physics PowerPoint What Are Calorimeters We will consider two types of calorimeters, ideal calorimeters, where the calorimeter does not absorb heat itself, and real calorimeters, where the calorimeter absorbs heat. It mainly consists of a metallic vessel made of materials which are good conductors of electricity such as copper and aluminium etc. Calorimetry is a branch of science concerned with measuring a body’s state in. What Are Calorimeters.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow What Are Calorimeters The magnitude of the temperature change depends on the amount. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. We will consider two types of calorimeters, ideal calorimeters, where the calorimeter does not absorb heat itself, and real calorimeters, where the calorimeter absorbs heat. A. What Are Calorimeters.
From saylordotorg.github.io
Calorimetry What Are Calorimeters Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. It mainly consists of a metallic vessel made of materials which are good conductors of electricity such as copper and aluminium etc. We will apply calorimetry to two different thermodynamic processes, that of transference of heat. What Are Calorimeters.
From thermonine92.blogspot.com
Thermochemistry Calorimeter What Are Calorimeters Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. The magnitude of the temperature change depends on the amount. Calorimeter, device for measuring the heat developed. What Are Calorimeters.
From stock.adobe.com
Vettoriale Stock illustration of chemistry and physics, Calorimeter What Are Calorimeters We will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at different temperatures, and that released or absorbed by. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its. What Are Calorimeters.
From www.laboratory-equipment.com
Laboratory Calorimeters from IKA What Are Calorimeters Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. We will. What Are Calorimeters.
From www.thoughtco.com
Calorimeter Definition in Chemistry What Are Calorimeters Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter is a device used for heat measurements necessary for calorimetry. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Chemists use calorimetry to determine. What Are Calorimeters.
From users.highland.edu
Calorimetry What Are Calorimeters We will consider two types of calorimeters, ideal calorimeters, where the calorimeter does not absorb heat itself, and real calorimeters, where the calorimeter absorbs heat. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry is a branch of science concerned with measuring a body’s state in terms of. What Are Calorimeters.
From www.laboratory-equipment.com
Laboratory Calorimeters from IKA What Are Calorimeters We will apply calorimetry to two different thermodynamic processes, that of transference of heat across objects at different temperatures, and that released or absorbed by. We will consider two types of calorimeters, ideal calorimeters, where the calorimeter does not absorb heat itself, and real calorimeters, where the calorimeter absorbs heat. Calorimeter, device for measuring the heat developed during a mechanical,. What Are Calorimeters.
From labsuppliesusa.com
Calorimeter Electric KLM Bio Scientific What Are Calorimeters Chemists use calorimetry to determine the amount of heat. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. It uses devices called calorimeters, which measure the change in temperature when a chemical reaction is carried out. Calorimetry is a branch of science concerned with measuring a body’s state in. What Are Calorimeters.