What Is Electron Sharing . Electrons shared in pure covalent bonds have an equal probability of being near each nucleus. Electron sharing and covalent bonds. What is a covalent chemical bond? Let us illustrate a covalent bond by using h atoms,. Each atom donates a single electron to the electron pair which is shared. When electrons are shared between two atoms, they make a bond called a covalent bond. Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. Electron sharing is the process by which two or more atoms combine to form a chemical bond by sharing pairs of electrons. It is this sharing of electrons that we refer to as a chemical bond,. In the case of cl 2, each atom starts off with. In a simple wooden model, two balls representing atoms.
from sphweb.bumc.bu.edu
What is a covalent chemical bond? Electron sharing is the process by which two or more atoms combine to form a chemical bond by sharing pairs of electrons. In a simple wooden model, two balls representing atoms. Each atom donates a single electron to the electron pair which is shared. When electrons are shared between two atoms, they make a bond called a covalent bond. Electrons shared in pure covalent bonds have an equal probability of being near each nucleus. Let us illustrate a covalent bond by using h atoms,. The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. Electron sharing and covalent bonds. Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms.
Basic Cell Biology
What Is Electron Sharing The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. Electrons shared in pure covalent bonds have an equal probability of being near each nucleus. What is a covalent chemical bond? In a simple wooden model, two balls representing atoms. It is this sharing of electrons that we refer to as a chemical bond,. The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. In the case of cl 2, each atom starts off with. When electrons are shared between two atoms, they make a bond called a covalent bond. Electron sharing and covalent bonds. Let us illustrate a covalent bond by using h atoms,. Electron sharing is the process by which two or more atoms combine to form a chemical bond by sharing pairs of electrons. Each atom donates a single electron to the electron pair which is shared.
From emedia.rmit.edu.au
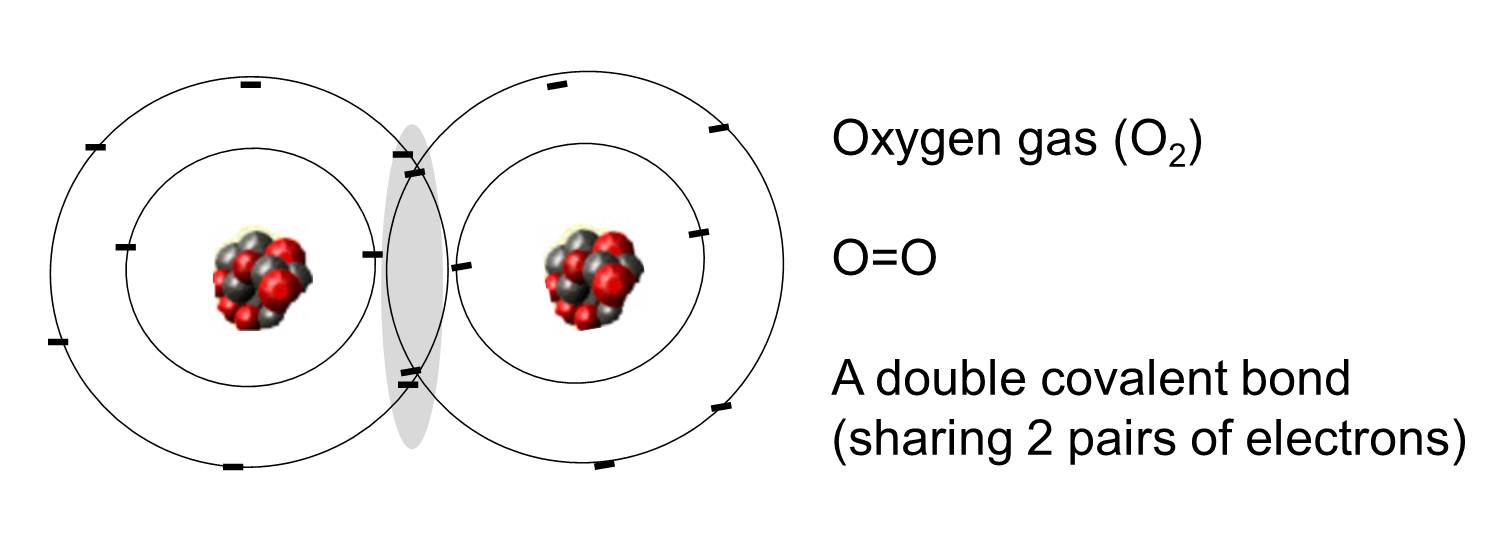
Covalent bonds Learning Lab What Is Electron Sharing Electron sharing and covalent bonds. It is this sharing of electrons that we refer to as a chemical bond,. Each atom donates a single electron to the electron pair which is shared. Electron sharing is the process by which two or more atoms combine to form a chemical bond by sharing pairs of electrons. Electrons shared in pure covalent bonds. What Is Electron Sharing.
From slidetodoc.com
Ionic Bonding The Octet Rule Noble gases are What Is Electron Sharing Let us illustrate a covalent bond by using h atoms,. Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. What is a covalent chemical bond? The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. Electron sharing is the process. What Is Electron Sharing.
From slideplayer.com
Bonds…. Covalent Bonds Chapter 5 sec. 3 Mr. Sapalicio ppt download What Is Electron Sharing Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. In the case of cl 2, each atom starts off with. Electron sharing and covalent bonds. The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. In a simple wooden model,. What Is Electron Sharing.
From www.slideserve.com
PPT Chapter 4 Formation of Compounds PowerPoint Presentation, free What Is Electron Sharing What is a covalent chemical bond? Electron sharing and covalent bonds. Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. When electrons are shared between two atoms, they make a bond called a covalent bond. Let us illustrate a covalent bond by using h atoms,. It is this sharing of. What Is Electron Sharing.
From www.slideserve.com
PPT CHEMICAL BONDS PowerPoint Presentation, free download ID6445592 What Is Electron Sharing In the case of cl 2, each atom starts off with. When electrons are shared between two atoms, they make a bond called a covalent bond. Let us illustrate a covalent bond by using h atoms,. Electron sharing is the process by which two or more atoms combine to form a chemical bond by sharing pairs of electrons. Electron sharing. What Is Electron Sharing.
From www.slideserve.com
PPT Chapter 19— CHEMICAL BONDS PowerPoint Presentation, free download What Is Electron Sharing In a simple wooden model, two balls representing atoms. Each atom donates a single electron to the electron pair which is shared. In the case of cl 2, each atom starts off with. The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. Covalent bond, in chemistry, the interatomic linkage. What Is Electron Sharing.
From slideplayer.com
Covalent Bonds Chapter 5 Section ppt download What Is Electron Sharing What is a covalent chemical bond? In a simple wooden model, two balls representing atoms. Electron sharing is the process by which two or more atoms combine to form a chemical bond by sharing pairs of electrons. Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. Electron sharing and covalent. What Is Electron Sharing.
From chem.libretexts.org
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts What Is Electron Sharing What is a covalent chemical bond? When electrons are shared between two atoms, they make a bond called a covalent bond. The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. Each atom donates a single electron to the electron pair which is shared. Electron sharing is the process by. What Is Electron Sharing.
From alevelchemistry.co.uk
Electrons Facts, Summary & Definition Chemistry Revision What Is Electron Sharing Each atom donates a single electron to the electron pair which is shared. It is this sharing of electrons that we refer to as a chemical bond,. Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. Electron sharing is the process by which two or more atoms combine to form. What Is Electron Sharing.
From www.slideserve.com
PPT Section 1 Atoms, Bonding, and the Periodic Table PowerPoint What Is Electron Sharing Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. Electron sharing is the process by which two or more atoms combine to form a chemical bond by sharing pairs of electrons. In a simple wooden model, two balls representing atoms. Electrons shared in pure covalent bonds have an equal probability. What Is Electron Sharing.
From slideplayer.com
Electron Sharing aww isn’t that nice ppt download What Is Electron Sharing Each atom donates a single electron to the electron pair which is shared. Electron sharing and covalent bonds. Let us illustrate a covalent bond by using h atoms,. Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. Electron sharing is the process by which two or more atoms combine to. What Is Electron Sharing.
From www.slideserve.com
PPT Chapter 12 Covalent Bonding PowerPoint Presentation, free What Is Electron Sharing The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. Each atom donates a single electron to the electron pair which is shared. Electrons shared in pure covalent bonds have an equal probability of being near each nucleus. Covalent bond, in chemistry, the interatomic linkage that results from the sharing. What Is Electron Sharing.
From slidetodoc.com
Covalent Bonds Molecules IV Electron Sharing Produces Molecules What Is Electron Sharing The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. In a simple wooden model, two balls representing atoms. Each atom donates a single electron to the electron pair which is shared. What is a covalent chemical bond? Covalent bond, in chemistry, the interatomic linkage that results from the sharing. What Is Electron Sharing.
From www.slideserve.com
PPT Section 1 Atoms, Bonding, and the Periodic Table PowerPoint What Is Electron Sharing Each atom donates a single electron to the electron pair which is shared. Electron sharing and covalent bonds. What is a covalent chemical bond? When electrons are shared between two atoms, they make a bond called a covalent bond. Electrons shared in pure covalent bonds have an equal probability of being near each nucleus. In a simple wooden model, two. What Is Electron Sharing.
From www.slideserve.com
PPT Chapter 5 Atoms & Bonding PowerPoint Presentation ID5979513 What Is Electron Sharing Electron sharing is the process by which two or more atoms combine to form a chemical bond by sharing pairs of electrons. Let us illustrate a covalent bond by using h atoms,. In a simple wooden model, two balls representing atoms. It is this sharing of electrons that we refer to as a chemical bond,. Electrons shared in pure covalent. What Is Electron Sharing.
From www.sliderbase.com
Chemical Bonds What Is Electron Sharing Each atom donates a single electron to the electron pair which is shared. In the case of cl 2, each atom starts off with. Electron sharing is the process by which two or more atoms combine to form a chemical bond by sharing pairs of electrons. Let us illustrate a covalent bond by using h atoms,. What is a covalent. What Is Electron Sharing.
From depositphotos.com
What Happens Atoms Bond Infographic Diagram Showing How Electrons What Is Electron Sharing It is this sharing of electrons that we refer to as a chemical bond,. In a simple wooden model, two balls representing atoms. In the case of cl 2, each atom starts off with. What is a covalent chemical bond? Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. The. What Is Electron Sharing.
From www.slideserve.com
PPT Atomic Timeline PowerPoint Presentation ID4726565 What Is Electron Sharing It is this sharing of electrons that we refer to as a chemical bond,. Electrons shared in pure covalent bonds have an equal probability of being near each nucleus. Electron sharing and covalent bonds. Each atom donates a single electron to the electron pair which is shared. Let us illustrate a covalent bond by using h atoms,. Covalent bond, in. What Is Electron Sharing.
From slideplayer.com
Covalent Bonds Chapter 5 Section ppt download What Is Electron Sharing What is a covalent chemical bond? In a simple wooden model, two balls representing atoms. When electrons are shared between two atoms, they make a bond called a covalent bond. Electron sharing and covalent bonds. Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. Let us illustrate a covalent bond. What Is Electron Sharing.
From www.slideserve.com
PPT Notes 53 Covalent Bonds PowerPoint Presentation, free download What Is Electron Sharing When electrons are shared between two atoms, they make a bond called a covalent bond. Electron sharing and covalent bonds. Electron sharing is the process by which two or more atoms combine to form a chemical bond by sharing pairs of electrons. Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two. What Is Electron Sharing.
From www.slideserve.com
PPT Chemistry of Life PowerPoint Presentation, free download ID4270176 What Is Electron Sharing Electrons shared in pure covalent bonds have an equal probability of being near each nucleus. Electron sharing is the process by which two or more atoms combine to form a chemical bond by sharing pairs of electrons. In a simple wooden model, two balls representing atoms. It is this sharing of electrons that we refer to as a chemical bond,.. What Is Electron Sharing.
From www.toppr.com
Draw electron dot representation for the formation of HCl What Is Electron Sharing Each atom donates a single electron to the electron pair which is shared. Let us illustrate a covalent bond by using h atoms,. When electrons are shared between two atoms, they make a bond called a covalent bond. In a simple wooden model, two balls representing atoms. Electron sharing is the process by which two or more atoms combine to. What Is Electron Sharing.
From www.slideserve.com
PPT The Octet Rule PowerPoint Presentation, free download ID2654863 What Is Electron Sharing Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. It is this sharing of electrons that we refer to as a chemical bond,. Electrons shared in pure covalent bonds have an equal probability of being near each nucleus. Electron sharing and covalent bonds. In the case of cl 2, each. What Is Electron Sharing.
From www.slideserve.com
PPT Life, Chemistry and Water PowerPoint Presentation, free download What Is Electron Sharing It is this sharing of electrons that we refer to as a chemical bond,. Each atom donates a single electron to the electron pair which is shared. Electrons shared in pure covalent bonds have an equal probability of being near each nucleus. When electrons are shared between two atoms, they make a bond called a covalent bond. Electron sharing is. What Is Electron Sharing.
From masterconceptsinchemistry.com
How hydrogen atoms share valence electrons to form covalent bond and What Is Electron Sharing The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. In a simple wooden model, two balls representing atoms. Electrons shared in pure covalent bonds have an equal probability of being near each nucleus. Let us illustrate a covalent bond by using h atoms,. Each atom donates a single electron. What Is Electron Sharing.
From www.pinterest.com
Related image Chemistry education, Teaching chemistry, Science chemistry What Is Electron Sharing Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. In the case of cl 2, each atom starts off with. Each atom donates a single electron to the electron pair. What Is Electron Sharing.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Forces Bind Atoms Together What Is Electron Sharing Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. When electrons are shared between two atoms, they make a bond called a covalent bond. In a simple wooden model, two balls representing atoms. Electrons shared in pure covalent bonds have an equal probability of being near each nucleus. It is. What Is Electron Sharing.
From www.slideserve.com
PPT 13 p. 229 in the book What electron configurations do atoms What Is Electron Sharing In a simple wooden model, two balls representing atoms. When electrons are shared between two atoms, they make a bond called a covalent bond. Each atom donates a single electron to the electron pair which is shared. What is a covalent chemical bond? It is this sharing of electrons that we refer to as a chemical bond,. Electron sharing and. What Is Electron Sharing.
From sphweb.bumc.bu.edu
Basic Cell Biology What Is Electron Sharing Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. In the case of cl 2, each atom starts off with. In a simple wooden model, two balls representing atoms. When electrons are shared between two atoms, they make a bond called a covalent bond. Electron sharing and covalent bonds. It. What Is Electron Sharing.
From www.researchgate.net
a) Schematic diagram of the electronsharing TCNQ molecules in the What Is Electron Sharing In the case of cl 2, each atom starts off with. Each atom donates a single electron to the electron pair which is shared. Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. In a simple wooden model, two balls representing atoms. When electrons are shared between two atoms, they. What Is Electron Sharing.
From philschatz.com
Chemical Bonds · Anatomy and Physiology What Is Electron Sharing Electrons shared in pure covalent bonds have an equal probability of being near each nucleus. In a simple wooden model, two balls representing atoms. In the case of cl 2, each atom starts off with. Each atom donates a single electron to the electron pair which is shared. The sharing of a pair of electrons represents a single covalent bond,. What Is Electron Sharing.
From mammothmemory.net
Covalent bonding is where atoms share electrons by bonding What Is Electron Sharing In a simple wooden model, two balls representing atoms. Electrons shared in pure covalent bonds have an equal probability of being near each nucleus. Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. Electron sharing and covalent bonds. Let us illustrate a covalent bond by using h atoms,. Each atom. What Is Electron Sharing.
From www.slideserve.com
PPT Supplement for Chapter 1 The Structure of Matter PowerPoint What Is Electron Sharing Let us illustrate a covalent bond by using h atoms,. Covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. Electrons shared in pure covalent bonds have an equal probability of being near each nucleus. The sharing of a pair of electrons represents a single covalent bond, usually just referred to. What Is Electron Sharing.
From klaxanybf.blob.core.windows.net
What Is Sharing Of Electrons at Stanley Killinger blog What Is Electron Sharing The sharing of a pair of electrons represents a single covalent bond, usually just referred to as a single bond. Electron sharing and covalent bonds. Electrons shared in pure covalent bonds have an equal probability of being near each nucleus. What is a covalent chemical bond? Electron sharing is the process by which two or more atoms combine to form. What Is Electron Sharing.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Forces Bind Atoms Together What Is Electron Sharing What is a covalent chemical bond? Let us illustrate a covalent bond by using h atoms,. In the case of cl 2, each atom starts off with. When electrons are shared between two atoms, they make a bond called a covalent bond. It is this sharing of electrons that we refer to as a chemical bond,. In a simple wooden. What Is Electron Sharing.