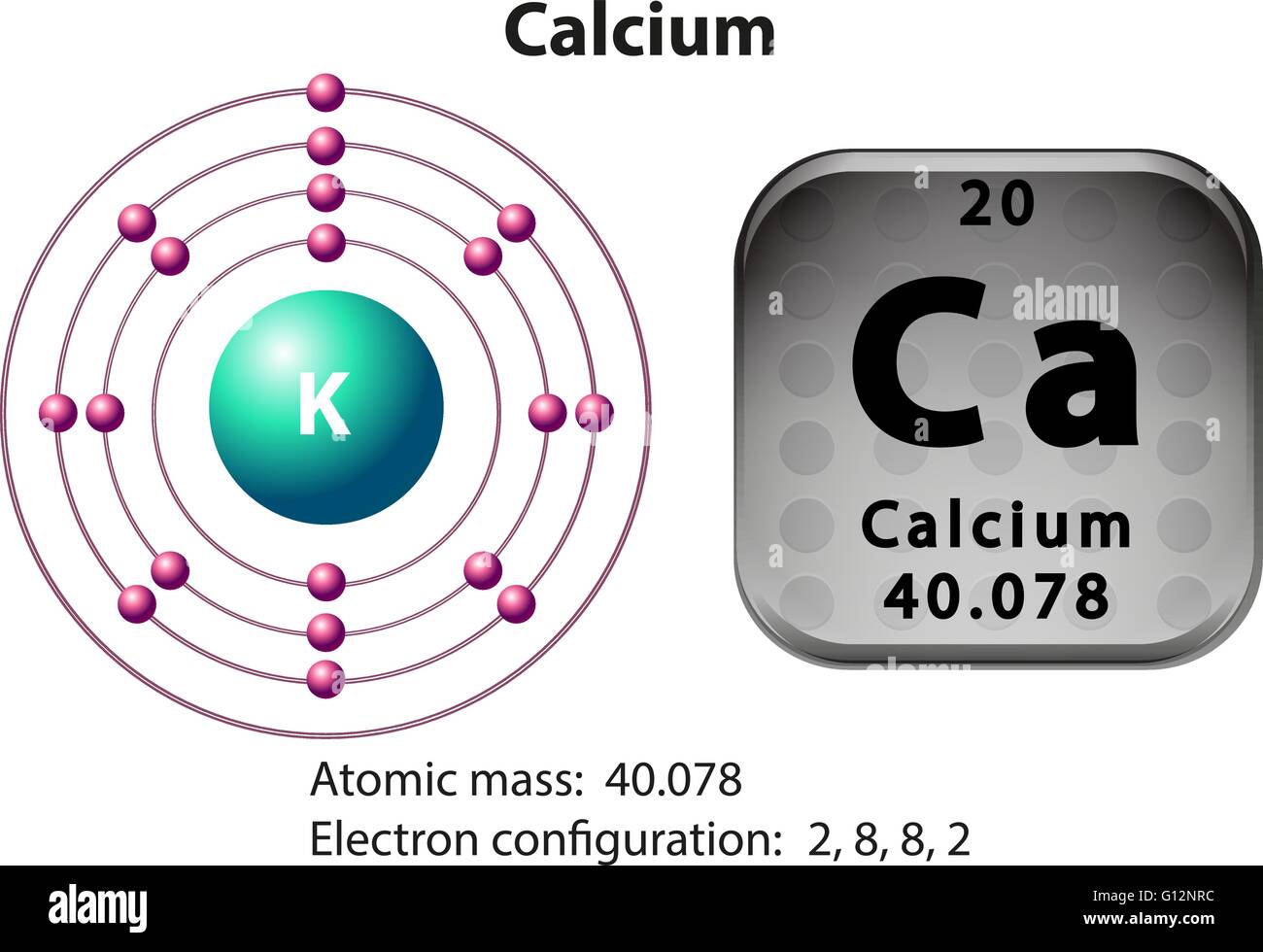
Electron Configuration For A Ca Ion . when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons, a 2+ charge, and an electron. Calcium’s atomic number is 20 which means that in a neutral calcium atom, in its nucleus there are 20 protons. the calcium has two valence electrons in its outer shell. find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. the electronic configuration of cations is assigned by removing electrons first in the outermost p orbital, followed by the s. What is the electron configuration of calcium. in this video we will write the electron configuration for ca2+, the calcium. when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons, a 2+ charge, and an electron. The electron configuration of a ca ion is 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2.
from ar.inspiredpencil.com
find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. the calcium has two valence electrons in its outer shell. the electronic configuration of cations is assigned by removing electrons first in the outermost p orbital, followed by the s. What is the electron configuration of calcium. in this video we will write the electron configuration for ca2+, the calcium. The electron configuration of a ca ion is 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2. Calcium’s atomic number is 20 which means that in a neutral calcium atom, in its nucleus there are 20 protons. when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons, a 2+ charge, and an electron. when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons, a 2+ charge, and an electron.
Calcium Electron Configuration Arrows
Electron Configuration For A Ca Ion What is the electron configuration of calcium. in this video we will write the electron configuration for ca2+, the calcium. the calcium has two valence electrons in its outer shell. find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons, a 2+ charge, and an electron. What is the electron configuration of calcium. Calcium’s atomic number is 20 which means that in a neutral calcium atom, in its nucleus there are 20 protons. The electron configuration of a ca ion is 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2. when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons, a 2+ charge, and an electron. the electronic configuration of cations is assigned by removing electrons first in the outermost p orbital, followed by the s.
From chem.libretexts.org
2.2 Electron Configurations Chemistry LibreTexts Electron Configuration For A Ca Ion in this video we will write the electron configuration for ca2+, the calcium. What is the electron configuration of calcium. Calcium’s atomic number is 20 which means that in a neutral calcium atom, in its nucleus there are 20 protons. when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons,. Electron Configuration For A Ca Ion.
From www.sciencephoto.com
Calcium electron configuration Stock Image C029/5027 Science Electron Configuration For A Ca Ion when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons, a 2+ charge, and an electron. find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. the electronic configuration of cations is assigned by removing electrons first in the outermost. Electron Configuration For A Ca Ion.
From iperiodictable.com
Calcium Electron Configuration Periodic Table Electron Configuration For A Ca Ion find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons, a 2+ charge, and an electron. in this video we will write the electron configuration for ca2+, the calcium. What. Electron Configuration For A Ca Ion.
From www.newtondesk.com
Calcium Ca (Element 20) of Periodic Table Elements FlashCards Electron Configuration For A Ca Ion when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons, a 2+ charge, and an electron. What is the electron configuration of calcium. when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons, a 2+ charge, and an electron. find the. Electron Configuration For A Ca Ion.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram Electron Configuration For A Ca Ion the calcium has two valence electrons in its outer shell. in this video we will write the electron configuration for ca2+, the calcium. when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons, a 2+ charge, and an electron. Calcium’s atomic number is 20 which means that in a. Electron Configuration For A Ca Ion.
From www.youtube.com
How to find Protons & Electrons for the Calcium ion (Ca 2+) YouTube Electron Configuration For A Ca Ion Calcium’s atomic number is 20 which means that in a neutral calcium atom, in its nucleus there are 20 protons. The electron configuration of a ca ion is 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2. find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. when a ca atom loses. Electron Configuration For A Ca Ion.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps Electron Configuration For A Ca Ion when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons, a 2+ charge, and an electron. the calcium has two valence electrons in its outer shell. when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons, a 2+ charge, and an. Electron Configuration For A Ca Ion.
From commons.wikimedia.org
FileElectron shell 020 calcium.png Electron Configuration For A Ca Ion Calcium’s atomic number is 20 which means that in a neutral calcium atom, in its nucleus there are 20 protons. when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons, a 2+ charge, and an electron. the electronic configuration of cations is assigned by removing electrons first in the outermost. Electron Configuration For A Ca Ion.
From www.youtube.com
Electron Configuration of Ions YouTube Electron Configuration For A Ca Ion Calcium’s atomic number is 20 which means that in a neutral calcium atom, in its nucleus there are 20 protons. find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons, a. Electron Configuration For A Ca Ion.
From valenceelectrons.com
Californium(Cf) Electron Configuration Details Explanation Electron Configuration For A Ca Ion in this video we will write the electron configuration for ca2+, the calcium. the calcium has two valence electrons in its outer shell. when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons, a 2+ charge, and an electron. What is the electron configuration of calcium. Calcium’s atomic number. Electron Configuration For A Ca Ion.
From iperiodictable.com
Orbital Diagram For Calcium (Ca) Calcium Electron Configuration Electron Configuration For A Ca Ion Calcium’s atomic number is 20 which means that in a neutral calcium atom, in its nucleus there are 20 protons. when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons, a 2+ charge, and an electron. when a ca atom loses both of its valence electrons, the result is a. Electron Configuration For A Ca Ion.
From www.thoughtco.com
Electron Configuration Chart Electron Configuration For A Ca Ion find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. the electronic configuration of cations is assigned by removing electrons first in the outermost p orbital, followed by the s. the calcium has two valence electrons in its outer shell. when a ca atom loses both of. Electron Configuration For A Ca Ion.
From www.dreamstime.com
Carbon Element 6 Electron Configuration Vector Illustration Diagram Electron Configuration For A Ca Ion The electron configuration of a ca ion is 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2. find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. Calcium’s atomic number is 20 which means that in a neutral calcium atom, in its nucleus there are 20 protons. when a ca atom loses. Electron Configuration For A Ca Ion.
From wirelistpursuings.z21.web.core.windows.net
Valence Electron Orbital Level Diagram Electron Configuration For A Ca Ion in this video we will write the electron configuration for ca2+, the calcium. the electronic configuration of cations is assigned by removing electrons first in the outermost p orbital, followed by the s. when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons, a 2+ charge, and an electron.. Electron Configuration For A Ca Ion.
From chemwiki.ucdavis.edu
Electronic Configurations Chemwiki Electron Configuration For A Ca Ion The electron configuration of a ca ion is 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2. find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons, a 2+ charge, and an electron. . Electron Configuration For A Ca Ion.
From www.slideserve.com
PPT How do atoms form ions? PowerPoint Presentation, free download Electron Configuration For A Ca Ion when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons, a 2+ charge, and an electron. Calcium’s atomic number is 20 which means that in a neutral calcium atom, in its nucleus there are 20 protons. when a ca atom loses both of its valence electrons, the result is a. Electron Configuration For A Ca Ion.
From ar.inspiredpencil.com
Electron Shell Diagram Calcium Electron Configuration For A Ca Ion The electron configuration of a ca ion is 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2. Calcium’s atomic number is 20 which means that in a neutral calcium atom, in its nucleus there are 20 protons. when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons, a 2+ charge, and an electron. . Electron Configuration For A Ca Ion.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps Electron Configuration For A Ca Ion in this video we will write the electron configuration for ca2+, the calcium. the calcium has two valence electrons in its outer shell. when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons, a 2+ charge, and an electron. What is the electron configuration of calcium. when a. Electron Configuration For A Ca Ion.
From valenceelectrons.com
Electron Configuration for Calcium (Ca, Ca2+ ion) Electron Configuration For A Ca Ion The electron configuration of a ca ion is 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2. What is the electron configuration of calcium. the electronic configuration of cations is assigned by removing electrons first in the outermost p orbital, followed by the s. the calcium has two valence electrons in its outer shell. when a ca atom loses both. Electron Configuration For A Ca Ion.
From www.youtube.com
CHEMISTRY 101 Electron configurations for ions YouTube Electron Configuration For A Ca Ion the electronic configuration of cations is assigned by removing electrons first in the outermost p orbital, followed by the s. in this video we will write the electron configuration for ca2+, the calcium. when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons, a 2+ charge, and an electron.. Electron Configuration For A Ca Ion.
From homedeso.vercel.app
Group 1 Periodic Table Electron Configuration Electron Configuration For A Ca Ion in this video we will write the electron configuration for ca2+, the calcium. What is the electron configuration of calcium. Calcium’s atomic number is 20 which means that in a neutral calcium atom, in its nucleus there are 20 protons. the calcium has two valence electrons in its outer shell. when a ca atom loses both of. Electron Configuration For A Ca Ion.
From material-properties.org
Calcium Protons Neutrons Electrons Electron Configuration Electron Configuration For A Ca Ion when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons, a 2+ charge, and an electron. find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. Calcium’s atomic number is 20 which means that in a neutral calcium atom, in its. Electron Configuration For A Ca Ion.
From periodictable.me
Calcium Electron Configuration (Ca) with Orbital Diagram Electron Configuration For A Ca Ion Calcium’s atomic number is 20 which means that in a neutral calcium atom, in its nucleus there are 20 protons. when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons, a 2+ charge, and an electron. in this video we will write the electron configuration for ca2+, the calcium. . Electron Configuration For A Ca Ion.
From www.youtube.com
Ca 2+ Electron Configuration (Calcium Ion) YouTube Electron Configuration For A Ca Ion the electronic configuration of cations is assigned by removing electrons first in the outermost p orbital, followed by the s. What is the electron configuration of calcium. Calcium’s atomic number is 20 which means that in a neutral calcium atom, in its nucleus there are 20 protons. when a ca atom loses both of its valence electrons, the. Electron Configuration For A Ca Ion.
From www.benjamin-mills.com
Electron arrangements Electron Configuration For A Ca Ion when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons, a 2+ charge, and an electron. What is the electron configuration of calcium. find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. the electronic configuration of cations is assigned. Electron Configuration For A Ca Ion.
From sciencenotes.org
List of Electron Configurations of Elements Electron Configuration For A Ca Ion The electron configuration of a ca ion is 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2. in this video we will write the electron configuration for ca2+, the calcium. the electronic configuration of cations is assigned by removing electrons first in the outermost p orbital, followed by the s. the calcium has two valence electrons in its outer shell.. Electron Configuration For A Ca Ion.
From giozhverl.blob.core.windows.net
Magnesium Long Electron Configuration at Michael Woods blog Electron Configuration For A Ca Ion in this video we will write the electron configuration for ca2+, the calcium. Calcium’s atomic number is 20 which means that in a neutral calcium atom, in its nucleus there are 20 protons. find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. when a ca atom loses. Electron Configuration For A Ca Ion.
From homedeso.vercel.app
Iron Periodic Table Electron Configuration Electron Configuration For A Ca Ion What is the electron configuration of calcium. find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. the electronic configuration of cations is assigned by removing electrons first in the outermost p orbital, followed by the s. The electron configuration of a ca ion is 1s^2 2s^2 2p^6 3s^2. Electron Configuration For A Ca Ion.
From animalia-life.club
Calcium Ion Lewis Dot Structure Electron Configuration For A Ca Ion when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons, a 2+ charge, and an electron. in this video we will write the electron configuration for ca2+, the calcium. when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons, a 2+. Electron Configuration For A Ca Ion.
From ar.inspiredpencil.com
Calcium Electron Configuration Arrows Electron Configuration For A Ca Ion What is the electron configuration of calcium. the electronic configuration of cations is assigned by removing electrons first in the outermost p orbital, followed by the s. find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. the calcium has two valence electrons in its outer shell. Calcium’s. Electron Configuration For A Ca Ion.
From www.slideserve.com
PPT Chapter Eight PowerPoint Presentation, free download ID1801337 Electron Configuration For A Ca Ion The electron configuration of a ca ion is 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2. when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons, a 2+ charge, and an electron. Calcium’s atomic number is 20 which means that in a neutral calcium atom, in its nucleus there are 20 protons. . Electron Configuration For A Ca Ion.
From kunduz.com
[ANSWERED] Write the full electron configuration for a Ca ion electron Electron Configuration For A Ca Ion the electronic configuration of cations is assigned by removing electrons first in the outermost p orbital, followed by the s. find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons,. Electron Configuration For A Ca Ion.
From studylib.net
Electron Configurations of Ions Electron Configuration For A Ca Ion What is the electron configuration of calcium. the electronic configuration of cations is assigned by removing electrons first in the outermost p orbital, followed by the s. Calcium’s atomic number is 20 which means that in a neutral calcium atom, in its nucleus there are 20 protons. when a ca atom loses both of its valence electrons, the. Electron Configuration For A Ca Ion.
From www.youtube.com
Electron configurations of ions Atomic structure and properties AP Electron Configuration For A Ca Ion when a ca atom loses both of its valence electrons, the result is a cation with 18 electrons, a 2+ charge, and an electron. in this video we will write the electron configuration for ca2+, the calcium. the calcium has two valence electrons in its outer shell. Calcium’s atomic number is 20 which means that in a. Electron Configuration For A Ca Ion.
From valenceelectrons.com
How to Write the Electron Configuration for Calcium (Ca)? Electron Configuration For A Ca Ion What is the electron configuration of calcium. The electron configuration of a ca ion is 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2. the calcium has two valence electrons in its outer shell. Calcium’s atomic number is 20 which means that in a neutral calcium atom, in its nucleus there are 20 protons. find the atomic number of nitrogen (7). Electron Configuration For A Ca Ion.