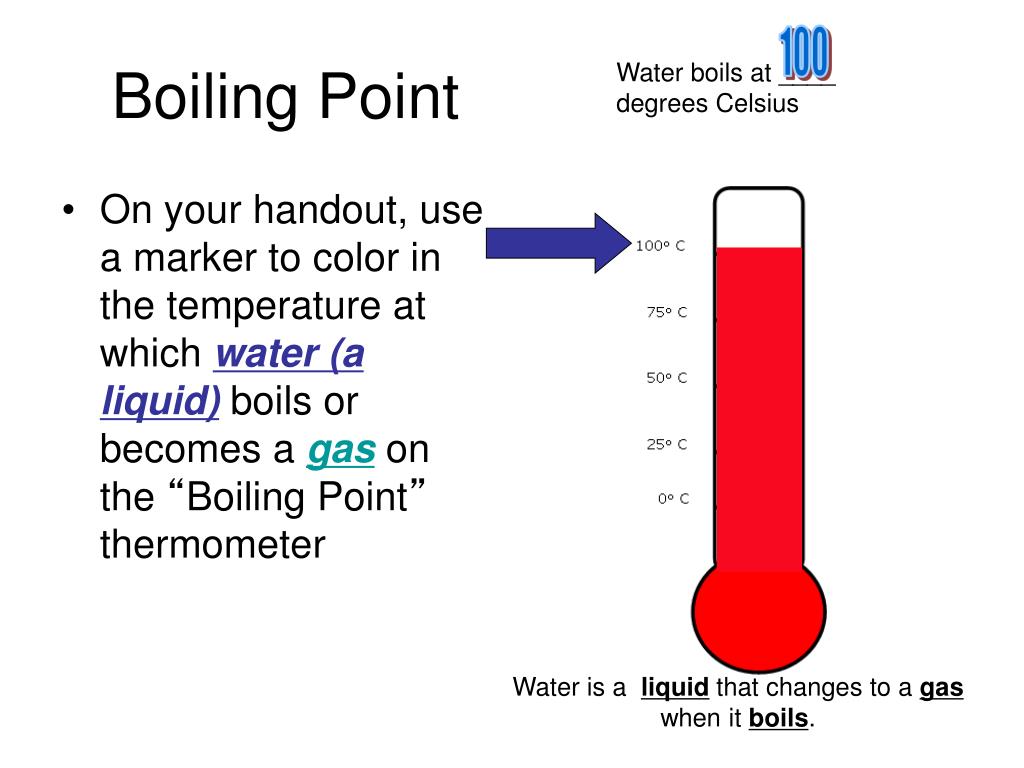
Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water . ( a ) both boiling point and freezing point. another effect because the changing of the intermolecular forces is the increasing of boiling temperature. Boiling point increases and freezing point decreases. what is the effect of the addition of sugar on the boiling and freezing points of water? adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making. Adding 1 gram of sugar, or any other substance that does not create. adding sugar to water increases the boiling point. Sugar + water mixture an eg. If 342g of cane sugar is dissolved in 1000 ml of water, the. the addition of one mole of sucrose (molecular compound) in one liter of water will raise the boiling point from 100. adding sugar to water will raise the boiling point and decrease the freezing point of water.
from exouuujdu.blob.core.windows.net
Adding 1 gram of sugar, or any other substance that does not create. adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making. adding sugar to water will raise the boiling point and decrease the freezing point of water. Sugar + water mixture an eg. adding sugar to water increases the boiling point. If 342g of cane sugar is dissolved in 1000 ml of water, the. what is the effect of the addition of sugar on the boiling and freezing points of water? another effect because the changing of the intermolecular forces is the increasing of boiling temperature. Boiling point increases and freezing point decreases. the addition of one mole of sucrose (molecular compound) in one liter of water will raise the boiling point from 100.
Is The Boiling Point Of Water Always 100 Degrees Celsius at Juanita Gafford blog
Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making. Sugar + water mixture an eg. adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making. what is the effect of the addition of sugar on the boiling and freezing points of water? ( a ) both boiling point and freezing point. Boiling point increases and freezing point decreases. adding sugar to water increases the boiling point. another effect because the changing of the intermolecular forces is the increasing of boiling temperature. adding sugar to water will raise the boiling point and decrease the freezing point of water. If 342g of cane sugar is dissolved in 1000 ml of water, the. the addition of one mole of sucrose (molecular compound) in one liter of water will raise the boiling point from 100. Adding 1 gram of sugar, or any other substance that does not create.
From dxojgfcke.blob.core.windows.net
What Is The Boiling Point And Freezing Point Of Water On Kelvin Scale at Grant Massey blog Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water ( a ) both boiling point and freezing point. Boiling point increases and freezing point decreases. Adding 1 gram of sugar, or any other substance that does not create. adding sugar to water increases the boiling point. adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making. If 342g of cane. Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.youtube.com
A 5 solution (by mass) of cane sugar in water has freezing point of 271K. Calculate the Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water ( a ) both boiling point and freezing point. adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making. adding sugar to water increases the boiling point. another effect because the changing of the intermolecular forces is the increasing of boiling temperature. the addition of one mole of sucrose. Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water.
From courses.lumenlearning.com
Colligative Properties Chemistry Atoms First Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water Boiling point increases and freezing point decreases. If 342g of cane sugar is dissolved in 1000 ml of water, the. adding sugar to water will raise the boiling point and decrease the freezing point of water. adding sugar to water increases the boiling point. another effect because the changing of the intermolecular forces is the increasing of. Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID3993444 Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water ( a ) both boiling point and freezing point. what is the effect of the addition of sugar on the boiling and freezing points of water? adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making. another effect because the changing of the intermolecular forces is the increasing of boiling. Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water.
From ar.inspiredpencil.com
Boiling Point Elevation Equation Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water If 342g of cane sugar is dissolved in 1000 ml of water, the. ( a ) both boiling point and freezing point. another effect because the changing of the intermolecular forces is the increasing of boiling temperature. the addition of one mole of sucrose (molecular compound) in one liter of water will raise the boiling point from 100.. Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.youtube.com
How to calculate new freezing and boiling point when solute is added. Colligative properties Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water another effect because the changing of the intermolecular forces is the increasing of boiling temperature. what is the effect of the addition of sugar on the boiling and freezing points of water? the addition of one mole of sucrose (molecular compound) in one liter of water will raise the boiling point from 100. Adding 1 gram of. Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water.
From guidemanualeruptivity.z14.web.core.windows.net
Chemistry Boiling Point Chart Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water the addition of one mole of sucrose (molecular compound) in one liter of water will raise the boiling point from 100. ( a ) both boiling point and freezing point. what is the effect of the addition of sugar on the boiling and freezing points of water? another effect because the changing of the intermolecular forces is. Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock Vector Illustration of Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water the addition of one mole of sucrose (molecular compound) in one liter of water will raise the boiling point from 100. ( a ) both boiling point and freezing point. another effect because the changing of the intermolecular forces is the increasing of boiling temperature. Boiling point increases and freezing point decreases. If 342g of cane sugar is. Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water.
From sciencenotes.org
What Is the Freezing Point of Water? Fahrenheit, Celsius, and Kelvin Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water what is the effect of the addition of sugar on the boiling and freezing points of water? Adding 1 gram of sugar, or any other substance that does not create. Boiling point increases and freezing point decreases. adding sugar to water will raise the boiling point and decrease the freezing point of water. ( a ) both boiling. Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water.
From dxojgfcke.blob.core.windows.net
What Is The Boiling Point And Freezing Point Of Water On Kelvin Scale at Grant Massey blog Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water what is the effect of the addition of sugar on the boiling and freezing points of water? Adding 1 gram of sugar, or any other substance that does not create. adding sugar to water increases the boiling point. adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making. If 342g. Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.scribd.com
How Salt And Sugar Affect The Boiling Point Of Water... By Aditya, Jojo, Zoe, And Nora Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water Sugar + water mixture an eg. adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making. Boiling point increases and freezing point decreases. If 342g of cane sugar is dissolved in 1000 ml of water, the. the addition of one mole of sucrose (molecular compound) in one liter of water will. Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.numerade.com
SOLVED The freezing point of a liquid will change when a solute is added. Explain how the Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water Boiling point increases and freezing point decreases. another effect because the changing of the intermolecular forces is the increasing of boiling temperature. Sugar + water mixture an eg. adding sugar to water increases the boiling point. If 342g of cane sugar is dissolved in 1000 ml of water, the. Adding 1 gram of sugar, or any other substance. Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water If 342g of cane sugar is dissolved in 1000 ml of water, the. Boiling point increases and freezing point decreases. another effect because the changing of the intermolecular forces is the increasing of boiling temperature. what is the effect of the addition of sugar on the boiling and freezing points of water? Sugar + water mixture an eg.. Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.numerade.com
SOLVED The freezing point of a liquid will change when a solute is added. Explain how the Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water ( a ) both boiling point and freezing point. Sugar + water mixture an eg. another effect because the changing of the intermolecular forces is the increasing of boiling temperature. what is the effect of the addition of sugar on the boiling and freezing points of water? the addition of one mole of sucrose (molecular compound) in. Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water.
From ar.inspiredpencil.com
Boiling Point Elevation Equation Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water adding sugar to water will raise the boiling point and decrease the freezing point of water. Sugar + water mixture an eg. ( a ) both boiling point and freezing point. adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making. If 342g of cane sugar is dissolved in 1000 ml. Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.slideserve.com
PPT Physical Properties of Water Boiling Point, Melting Point and Freezing Point PowerPoint Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water Adding 1 gram of sugar, or any other substance that does not create. adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making. If 342g of cane sugar is dissolved in 1000 ml of water, the. what is the effect of the addition of sugar on the boiling and freezing points. Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.gettyimages.ie
Freezing And Boiling Points In Celsius And Fahrenheit HighRes Vector Graphic Getty Images Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water Adding 1 gram of sugar, or any other substance that does not create. adding sugar to water will raise the boiling point and decrease the freezing point of water. adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making. the addition of one mole of sucrose (molecular compound) in one. Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water.
From stock.adobe.com
Boiling and Evaporation, Freezing and Melting Points of Water. Stock Vector Adobe Stock Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water adding sugar to water increases the boiling point. Sugar + water mixture an eg. adding sugar to water will raise the boiling point and decrease the freezing point of water. what is the effect of the addition of sugar on the boiling and freezing points of water? Boiling point increases and freezing point decreases. Adding 1 gram. Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.researchgate.net
Effect of GBFS addition on boiling water absorption. Download Scientific Diagram Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water adding sugar to water will raise the boiling point and decrease the freezing point of water. what is the effect of the addition of sugar on the boiling and freezing points of water? another effect because the changing of the intermolecular forces is the increasing of boiling temperature. adding sugar to water lowers the freezing point. Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water.
From chemistry.stackexchange.com
thermodynamics Why is the boiling point of sugarcane juice lower than the boiling point of Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water what is the effect of the addition of sugar on the boiling and freezing points of water? another effect because the changing of the intermolecular forces is the increasing of boiling temperature. Adding 1 gram of sugar, or any other substance that does not create. ( a ) both boiling point and freezing point. Boiling point increases and. Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.studocu.com
Boiling Point Elevation or Freezing Point Depression Boiling Point Elevation/ Freezing Point Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water If 342g of cane sugar is dissolved in 1000 ml of water, the. Sugar + water mixture an eg. adding sugar to water increases the boiling point. another effect because the changing of the intermolecular forces is the increasing of boiling temperature. the addition of one mole of sucrose (molecular compound) in one liter of water will. Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.pinterest.com
Boiling Point/Freezing/ Melting Point AnchorChart Teaching science, Science lessons, Science Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water Adding 1 gram of sugar, or any other substance that does not create. adding sugar to water increases the boiling point. Boiling point increases and freezing point decreases. adding sugar to water will raise the boiling point and decrease the freezing point of water. If 342g of cane sugar is dissolved in 1000 ml of water, the. . Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.toppr.com
Calculate the freezing point and the boiling point at 1 atmosphere of a solution containing 30 g Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water Boiling point increases and freezing point decreases. Sugar + water mixture an eg. adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making. If 342g of cane sugar is dissolved in 1000 ml of water, the. adding sugar to water will raise the boiling point and decrease the freezing point of. Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.youtube.com
Melting Point, Boiling Point and Freezing Point Chemistry YouTube Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water adding sugar to water will raise the boiling point and decrease the freezing point of water. ( a ) both boiling point and freezing point. the addition of one mole of sucrose (molecular compound) in one liter of water will raise the boiling point from 100. adding sugar to water increases the boiling point. what is. Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water ( a ) both boiling point and freezing point. If 342g of cane sugar is dissolved in 1000 ml of water, the. adding sugar to water will raise the boiling point and decrease the freezing point of water. another effect because the changing of the intermolecular forces is the increasing of boiling temperature. adding sugar to water. Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.numerade.com
SOLVED For a 0.50 molal solution of sucrose in water, calculate the freezing point and the Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water the addition of one mole of sucrose (molecular compound) in one liter of water will raise the boiling point from 100. adding sugar to water increases the boiling point. Sugar + water mixture an eg. Adding 1 gram of sugar, or any other substance that does not create. If 342g of cane sugar is dissolved in 1000 ml. Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water what is the effect of the addition of sugar on the boiling and freezing points of water? Adding 1 gram of sugar, or any other substance that does not create. ( a ) both boiling point and freezing point. If 342g of cane sugar is dissolved in 1000 ml of water, the. the addition of one mole of. Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water.
From dxojgfcke.blob.core.windows.net
What Is The Boiling Point And Freezing Point Of Water On Kelvin Scale at Grant Massey blog Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water If 342g of cane sugar is dissolved in 1000 ml of water, the. adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making. Sugar + water mixture an eg. adding sugar to water will raise the boiling point and decrease the freezing point of water. Boiling point increases and freezing point. Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water.
From ksa.mytutorsource.com
Water Freezing Point Definition, Factors Affecting It & Supercooled Water! MTS Blog Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water the addition of one mole of sucrose (molecular compound) in one liter of water will raise the boiling point from 100. what is the effect of the addition of sugar on the boiling and freezing points of water? another effect because the changing of the intermolecular forces is the increasing of boiling temperature. adding sugar to. Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water.
From inspiritvr.com
Boiling Point Elevation and Freezing Point Depression Study Guide Inspirit Learning Inc Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water Sugar + water mixture an eg. adding sugar to water lowers the freezing point because the sugar molecules prevent the water from making. Boiling point increases and freezing point decreases. adding sugar to water will raise the boiling point and decrease the freezing point of water. another effect because the changing of the intermolecular forces is the. Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water.
From www.engineeringtoolbox.com
Sugar Solubility in Water Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water adding sugar to water will raise the boiling point and decrease the freezing point of water. adding sugar to water increases the boiling point. ( a ) both boiling point and freezing point. the addition of one mole of sucrose (molecular compound) in one liter of water will raise the boiling point from 100. Sugar + water. Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water.
From mavink.com
Ethanol Water Freezing Point Chart Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water another effect because the changing of the intermolecular forces is the increasing of boiling temperature. Adding 1 gram of sugar, or any other substance that does not create. Sugar + water mixture an eg. the addition of one mole of sucrose (molecular compound) in one liter of water will raise the boiling point from 100. ( a ). Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water.
From dxojgfcke.blob.core.windows.net
What Is The Boiling Point And Freezing Point Of Water On Kelvin Scale at Grant Massey blog Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water ( a ) both boiling point and freezing point. the addition of one mole of sucrose (molecular compound) in one liter of water will raise the boiling point from 100. Adding 1 gram of sugar, or any other substance that does not create. what is the effect of the addition of sugar on the boiling and freezing points. Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water.
From slideplayer.com
Solutions. ppt download Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water Adding 1 gram of sugar, or any other substance that does not create. what is the effect of the addition of sugar on the boiling and freezing points of water? ( a ) both boiling point and freezing point. Boiling point increases and freezing point decreases. the addition of one mole of sucrose (molecular compound) in one liter. Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water.
From exouuujdu.blob.core.windows.net
Is The Boiling Point Of Water Always 100 Degrees Celsius at Juanita Gafford blog Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water Sugar + water mixture an eg. ( a ) both boiling point and freezing point. Adding 1 gram of sugar, or any other substance that does not create. what is the effect of the addition of sugar on the boiling and freezing points of water? Boiling point increases and freezing point decreases. the addition of one mole of. Q1 What Is The Effect Of The Addition Of Sugar On The Boiling And Freezing Point Of Water.