Indicator For Edta Titrations Against Caco3 . Calcium ions can be analyzed. The endpoint of an edta titration is determined with a metallochromic indicator. These indicators are complexing agents that change color when. In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium carbonate, caco3. Edta titration for determination of calcium and magnesium. The endpoint of an edta titration is determined with a metallochromic indicator. The overall procedure to be used involves the standardization of an edta solution by titration with a known amount of calcium followed by. You will then use the edta solution to. These indicators are complexing agents that change color when. In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium carbonate, caco3. Before attempting this experiment, you may need to consult the section in the. Eriochrome black t cannot be used as an indicator for the titration of calcium with edta, since it forms too weak a complex with calcium to give a sharp end point. In the lab 1 ppm caco3 is expressed as 1 mg caco3 per 1 liter of sample or ppm is mg caco3 per l of sample.
from www.gopracticals.com
You will then use the edta solution to. The endpoint of an edta titration is determined with a metallochromic indicator. These indicators are complexing agents that change color when. Edta titration for determination of calcium and magnesium. These indicators are complexing agents that change color when. In the lab 1 ppm caco3 is expressed as 1 mg caco3 per 1 liter of sample or ppm is mg caco3 per l of sample. Eriochrome black t cannot be used as an indicator for the titration of calcium with edta, since it forms too weak a complex with calcium to give a sharp end point. The overall procedure to be used involves the standardization of an edta solution by titration with a known amount of calcium followed by. The endpoint of an edta titration is determined with a metallochromic indicator. Before attempting this experiment, you may need to consult the section in the.
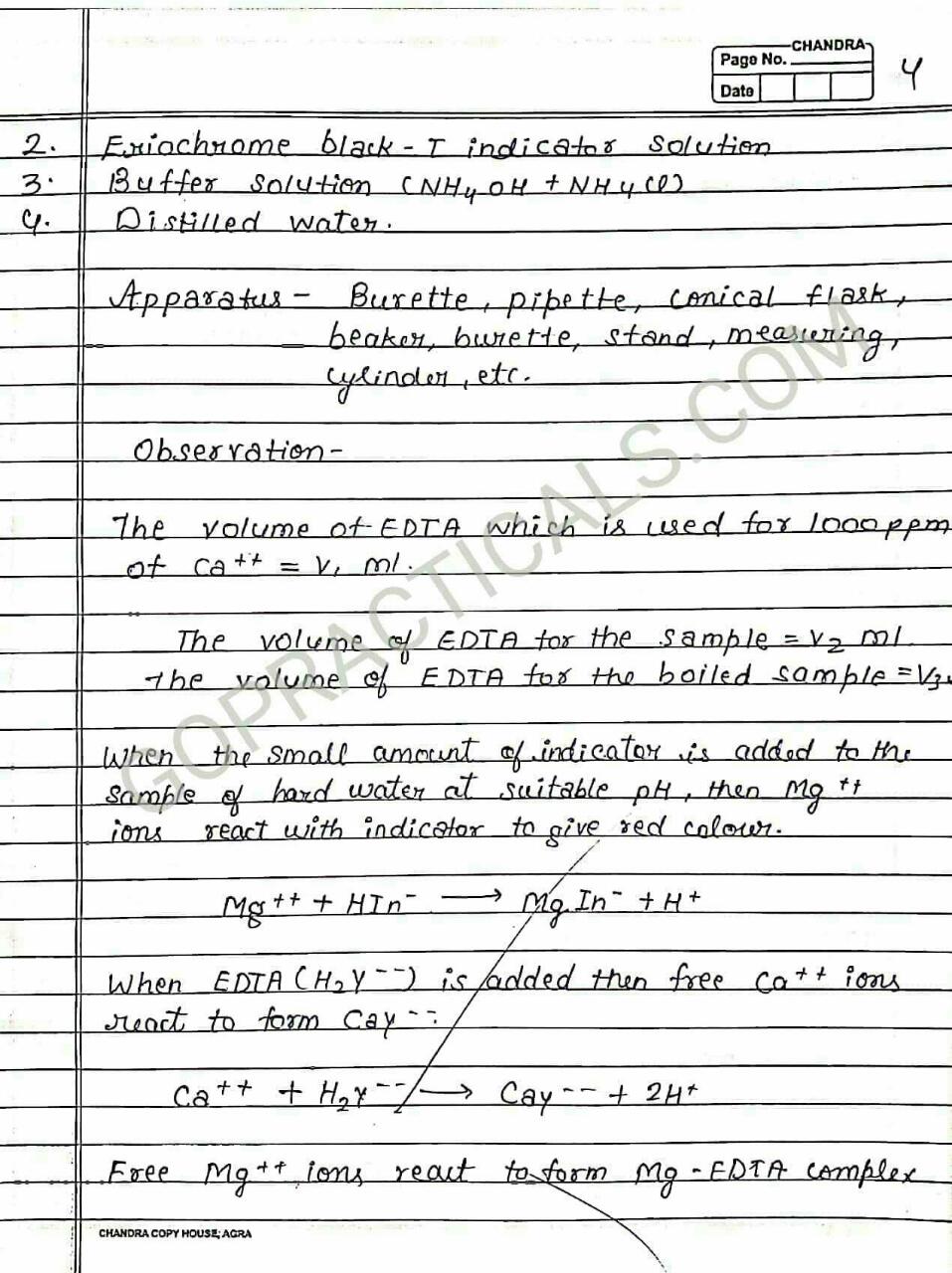
To Determine total hardness of Water sample in terms of Caco3 by EDTA
Indicator For Edta Titrations Against Caco3 In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium carbonate, caco3. Before attempting this experiment, you may need to consult the section in the. In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium carbonate, caco3. These indicators are complexing agents that change color when. Calcium ions can be analyzed. The overall procedure to be used involves the standardization of an edta solution by titration with a known amount of calcium followed by. These indicators are complexing agents that change color when. The endpoint of an edta titration is determined with a metallochromic indicator. Eriochrome black t cannot be used as an indicator for the titration of calcium with edta, since it forms too weak a complex with calcium to give a sharp end point. Edta titration for determination of calcium and magnesium. You will then use the edta solution to. The endpoint of an edta titration is determined with a metallochromic indicator. In the lab 1 ppm caco3 is expressed as 1 mg caco3 per 1 liter of sample or ppm is mg caco3 per l of sample. In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium carbonate, caco3.
From www.bartleby.com
Answered 1.Write the equation for reaction… bartleby Indicator For Edta Titrations Against Caco3 In the lab 1 ppm caco3 is expressed as 1 mg caco3 per 1 liter of sample or ppm is mg caco3 per l of sample. You will then use the edta solution to. Calcium ions can be analyzed. Before attempting this experiment, you may need to consult the section in the. The endpoint of an edta titration is determined. Indicator For Edta Titrations Against Caco3.
From www.numerade.com
Please answer these questions. It's urgent. Assignment Hardness Indicator For Edta Titrations Against Caco3 In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium carbonate, caco3. These indicators are complexing agents that change color when. You will then use the edta solution to. The endpoint of an edta titration is determined with a metallochromic indicator. Edta titration for determination of calcium and magnesium. Calcium ions. Indicator For Edta Titrations Against Caco3.
From www.bartleby.com
Answered Mass of zinc = 1.050 g. Calculate the… bartleby Indicator For Edta Titrations Against Caco3 Edta titration for determination of calcium and magnesium. These indicators are complexing agents that change color when. Eriochrome black t cannot be used as an indicator for the titration of calcium with edta, since it forms too weak a complex with calcium to give a sharp end point. The endpoint of an edta titration is determined with a metallochromic indicator.. Indicator For Edta Titrations Against Caco3.
From www.bartleby.com
Answered Which indicator is used in the… bartleby Indicator For Edta Titrations Against Caco3 These indicators are complexing agents that change color when. In the lab 1 ppm caco3 is expressed as 1 mg caco3 per 1 liter of sample or ppm is mg caco3 per l of sample. In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium carbonate, caco3. Before attempting this experiment,. Indicator For Edta Titrations Against Caco3.
From mmerevise.co.uk
pH Curves Questions and Revision MME Indicator For Edta Titrations Against Caco3 The overall procedure to be used involves the standardization of an edta solution by titration with a known amount of calcium followed by. These indicators are complexing agents that change color when. Before attempting this experiment, you may need to consult the section in the. In this experiment you will standardize a solution of edta by titration against a standard. Indicator For Edta Titrations Against Caco3.
From www.slideserve.com
PPT EDTA Titrations PowerPoint Presentation, free download ID1079499 Indicator For Edta Titrations Against Caco3 In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium carbonate, caco3. The overall procedure to be used involves the standardization of an edta solution by titration with a known amount of calcium followed by. These indicators are complexing agents that change color when. Calcium ions can be analyzed. The endpoint. Indicator For Edta Titrations Against Caco3.
From gioteyspe.blob.core.windows.net
Indicator Used For Complexometric Titrations at Jerlene Powell blog Indicator For Edta Titrations Against Caco3 In the lab 1 ppm caco3 is expressed as 1 mg caco3 per 1 liter of sample or ppm is mg caco3 per l of sample. These indicators are complexing agents that change color when. In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium carbonate, caco3. Calcium ions can be. Indicator For Edta Titrations Against Caco3.
From collegedunia.com
Complexometric Titration EDTA, Types & Applications Indicator For Edta Titrations Against Caco3 In the lab 1 ppm caco3 is expressed as 1 mg caco3 per 1 liter of sample or ppm is mg caco3 per l of sample. Eriochrome black t cannot be used as an indicator for the titration of calcium with edta, since it forms too weak a complex with calcium to give a sharp end point. The endpoint of. Indicator For Edta Titrations Against Caco3.
From www.numerade.com
SOLVED Titration of Cal+ and Mgl in a 50.00mL sample of hard water Indicator For Edta Titrations Against Caco3 Before attempting this experiment, you may need to consult the section in the. In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium carbonate, caco3. You will then use the edta solution to. Eriochrome black t cannot be used as an indicator for the titration of calcium with edta, since it. Indicator For Edta Titrations Against Caco3.
From www.studocu.com
EDTA Titration for Determination of calcium and magnesium In this Indicator For Edta Titrations Against Caco3 The endpoint of an edta titration is determined with a metallochromic indicator. You will then use the edta solution to. In the lab 1 ppm caco3 is expressed as 1 mg caco3 per 1 liter of sample or ppm is mg caco3 per l of sample. Eriochrome black t cannot be used as an indicator for the titration of calcium. Indicator For Edta Titrations Against Caco3.
From www.vrogue.co
Modelling An Edta Titration Curve Youtube vrogue.co Indicator For Edta Titrations Against Caco3 These indicators are complexing agents that change color when. Eriochrome black t cannot be used as an indicator for the titration of calcium with edta, since it forms too weak a complex with calcium to give a sharp end point. Edta titration for determination of calcium and magnesium. Before attempting this experiment, you may need to consult the section in. Indicator For Edta Titrations Against Caco3.
From www.gopracticals.com
To Determine total hardness of Water sample in terms of Caco3 by EDTA Indicator For Edta Titrations Against Caco3 You will then use the edta solution to. Eriochrome black t cannot be used as an indicator for the titration of calcium with edta, since it forms too weak a complex with calcium to give a sharp end point. In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium carbonate, caco3.. Indicator For Edta Titrations Against Caco3.
From www.vrogue.co
Why Is Phenolphthalein A Good Indicator For Acid Base vrogue.co Indicator For Edta Titrations Against Caco3 These indicators are complexing agents that change color when. You will then use the edta solution to. In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium carbonate, caco3. In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium carbonate, caco3. Eriochrome. Indicator For Edta Titrations Against Caco3.
From www.gopracticals.com
To Determine total hardness of Water sample in terms of Caco3 by EDTA Indicator For Edta Titrations Against Caco3 Eriochrome black t cannot be used as an indicator for the titration of calcium with edta, since it forms too weak a complex with calcium to give a sharp end point. These indicators are complexing agents that change color when. Edta titration for determination of calcium and magnesium. In this experiment you will standardize a solution of edta by titration. Indicator For Edta Titrations Against Caco3.
From www.numerade.com
SOLVED What is a complexometric indicator? What is complexometric Indicator For Edta Titrations Against Caco3 In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium carbonate, caco3. Eriochrome black t cannot be used as an indicator for the titration of calcium with edta, since it forms too weak a complex with calcium to give a sharp end point. These indicators are complexing agents that change color. Indicator For Edta Titrations Against Caco3.
From dokumen.tips
(PDF) EDTA Titration Calculations DOKUMEN.TIPS Indicator For Edta Titrations Against Caco3 These indicators are complexing agents that change color when. In the lab 1 ppm caco3 is expressed as 1 mg caco3 per 1 liter of sample or ppm is mg caco3 per l of sample. Before attempting this experiment, you may need to consult the section in the. The endpoint of an edta titration is determined with a metallochromic indicator.. Indicator For Edta Titrations Against Caco3.
From www.youtube.com
Determination of Hardness by EDTA Method YouTube Indicator For Edta Titrations Against Caco3 These indicators are complexing agents that change color when. The endpoint of an edta titration is determined with a metallochromic indicator. Calcium ions can be analyzed. You will then use the edta solution to. In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium carbonate, caco3. Before attempting this experiment, you. Indicator For Edta Titrations Against Caco3.
From mungfali.com
EDTA Titration Curve Indicator For Edta Titrations Against Caco3 Eriochrome black t cannot be used as an indicator for the titration of calcium with edta, since it forms too weak a complex with calcium to give a sharp end point. Before attempting this experiment, you may need to consult the section in the. You will then use the edta solution to. The endpoint of an edta titration is determined. Indicator For Edta Titrations Against Caco3.
From letitsnowglobe.co.uk
Titration procedure pdf Indicator For Edta Titrations Against Caco3 Edta titration for determination of calcium and magnesium. The endpoint of an edta titration is determined with a metallochromic indicator. The endpoint of an edta titration is determined with a metallochromic indicator. Calcium ions can be analyzed. The overall procedure to be used involves the standardization of an edta solution by titration with a known amount of calcium followed by.. Indicator For Edta Titrations Against Caco3.
From www.youtube.com
Calcium and Magnesium ion concentration determination with EDTA Indicator For Edta Titrations Against Caco3 In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium carbonate, caco3. The overall procedure to be used involves the standardization of an edta solution by titration with a known amount of calcium followed by. Eriochrome black t cannot be used as an indicator for the titration of calcium with edta,. Indicator For Edta Titrations Against Caco3.
From www.gopracticals.com
To Determine total hardness of Water sample in terms of Caco3 by EDTA Indicator For Edta Titrations Against Caco3 In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium carbonate, caco3. Edta titration for determination of calcium and magnesium. Eriochrome black t cannot be used as an indicator for the titration of calcium with edta, since it forms too weak a complex with calcium to give a sharp end point.. Indicator For Edta Titrations Against Caco3.
From www.numerade.com
SOLVED 88. Calcium chloride can be analyzed by mercurimetric titration Indicator For Edta Titrations Against Caco3 The endpoint of an edta titration is determined with a metallochromic indicator. Calcium ions can be analyzed. In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium carbonate, caco3. These indicators are complexing agents that change color when. The overall procedure to be used involves the standardization of an edta solution. Indicator For Edta Titrations Against Caco3.
From chrominfo.blogspot.com
Chrominfo Indicators of complexometric titration Indicator For Edta Titrations Against Caco3 Calcium ions can be analyzed. Edta titration for determination of calcium and magnesium. These indicators are complexing agents that change color when. In the lab 1 ppm caco3 is expressed as 1 mg caco3 per 1 liter of sample or ppm is mg caco3 per l of sample. The overall procedure to be used involves the standardization of an edta. Indicator For Edta Titrations Against Caco3.
From solutionpharmacy.in
Complexometric Titration Solution Parmacy Indicator For Edta Titrations Against Caco3 The endpoint of an edta titration is determined with a metallochromic indicator. In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium carbonate, caco3. Edta titration for determination of calcium and magnesium. The endpoint of an edta titration is determined with a metallochromic indicator. Before attempting this experiment, you may need. Indicator For Edta Titrations Against Caco3.
From www.slideshare.net
Complexometric titration Indicator For Edta Titrations Against Caco3 In the lab 1 ppm caco3 is expressed as 1 mg caco3 per 1 liter of sample or ppm is mg caco3 per l of sample. In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium carbonate, caco3. You will then use the edta solution to. Edta titration for determination of. Indicator For Edta Titrations Against Caco3.
From www.vrogue.co
Modelling An Edta Titration Curve Youtube vrogue.co Indicator For Edta Titrations Against Caco3 The endpoint of an edta titration is determined with a metallochromic indicator. These indicators are complexing agents that change color when. Edta titration for determination of calcium and magnesium. The endpoint of an edta titration is determined with a metallochromic indicator. Eriochrome black t cannot be used as an indicator for the titration of calcium with edta, since it forms. Indicator For Edta Titrations Against Caco3.
From www.numerade.com
SOLVED Title Experiment Determination of Hardness of Water by EDTA Indicator For Edta Titrations Against Caco3 Calcium ions can be analyzed. The endpoint of an edta titration is determined with a metallochromic indicator. The endpoint of an edta titration is determined with a metallochromic indicator. The overall procedure to be used involves the standardization of an edta solution by titration with a known amount of calcium followed by. You will then use the edta solution to.. Indicator For Edta Titrations Against Caco3.
From www.numerade.com
SOLVED What is a complexometric indicator? What is complexometric Indicator For Edta Titrations Against Caco3 The overall procedure to be used involves the standardization of an edta solution by titration with a known amount of calcium followed by. These indicators are complexing agents that change color when. You will then use the edta solution to. In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium carbonate,. Indicator For Edta Titrations Against Caco3.
From www.gopracticals.com
To Determine total hardness of Water sample in terms of Caco3 by EDTA Indicator For Edta Titrations Against Caco3 Before attempting this experiment, you may need to consult the section in the. In the lab 1 ppm caco3 is expressed as 1 mg caco3 per 1 liter of sample or ppm is mg caco3 per l of sample. Calcium ions can be analyzed. The overall procedure to be used involves the standardization of an edta solution by titration with. Indicator For Edta Titrations Against Caco3.
From www.youtube.com
Total Water Hardness using EDTA Titration YouTube Indicator For Edta Titrations Against Caco3 You will then use the edta solution to. The overall procedure to be used involves the standardization of an edta solution by titration with a known amount of calcium followed by. Before attempting this experiment, you may need to consult the section in the. The endpoint of an edta titration is determined with a metallochromic indicator. The endpoint of an. Indicator For Edta Titrations Against Caco3.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog Indicator For Edta Titrations Against Caco3 In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium carbonate, caco3. In the lab 1 ppm caco3 is expressed as 1 mg caco3 per 1 liter of sample or ppm is mg caco3 per l of sample. The endpoint of an edta titration is determined with a metallochromic indicator. You. Indicator For Edta Titrations Against Caco3.
From www.gopracticals.com
To Determine total hardness of Water sample in terms of Caco3 by EDTA Indicator For Edta Titrations Against Caco3 You will then use the edta solution to. The overall procedure to be used involves the standardization of an edta solution by titration with a known amount of calcium followed by. In the lab 1 ppm caco3 is expressed as 1 mg caco3 per 1 liter of sample or ppm is mg caco3 per l of sample. These indicators are. Indicator For Edta Titrations Against Caco3.
From www.gopracticals.com
To Determine total hardness of Water sample in terms of Caco3 by EDTA Indicator For Edta Titrations Against Caco3 In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium carbonate, caco3. These indicators are complexing agents that change color when. Eriochrome black t cannot be used as an indicator for the titration of calcium with edta, since it forms too weak a complex with calcium to give a sharp end. Indicator For Edta Titrations Against Caco3.
From www.numerade.com
SOLVED 5. 0.28gm of CaCO3 was dissolved in HCl and the solution made Indicator For Edta Titrations Against Caco3 The endpoint of an edta titration is determined with a metallochromic indicator. These indicators are complexing agents that change color when. In this experiment you will standardize a solution of edta by titration against a standard solution made from calcium carbonate, caco3. In the lab 1 ppm caco3 is expressed as 1 mg caco3 per 1 liter of sample or. Indicator For Edta Titrations Against Caco3.
From www.numerade.com
SOLVED In determining the water hardness in a lake nearby the Duay Indicator For Edta Titrations Against Caco3 These indicators are complexing agents that change color when. The endpoint of an edta titration is determined with a metallochromic indicator. The overall procedure to be used involves the standardization of an edta solution by titration with a known amount of calcium followed by. Calcium ions can be analyzed. In this experiment you will standardize a solution of edta by. Indicator For Edta Titrations Against Caco3.