Evaporation Effects On Intermolecular Forces . Therefore, we can compare the relative strengths of the imfs of the compounds to predict their relative boiling points. Intermolecular forces affect how molecules interact with each other, including with other molecules of themselves. in order for a liquid molecule to escape into the gas state, the molecule must have enough kinetic energy to overcome the intermolecular attractive forces in the liquid. ideally, a student should be able to construct and then use a structure (by understanding that the shape and. the larger the intermolecular forces in a compound, the slower its evaporation rate. effects of intermolecular forces. Recall that a given liquid sample will have molecules with a Intermolecular forces (imfs) can be used to predict relative boiling points. water evaporation induces intermolecular forces that build a crosslinking reaction gradient from the. The stronger the imfs, the lower the vapor pressure of the substance and the higher the boiling point.
from www.chegg.com
ideally, a student should be able to construct and then use a structure (by understanding that the shape and. Recall that a given liquid sample will have molecules with a The stronger the imfs, the lower the vapor pressure of the substance and the higher the boiling point. Intermolecular forces affect how molecules interact with each other, including with other molecules of themselves. the larger the intermolecular forces in a compound, the slower its evaporation rate. water evaporation induces intermolecular forces that build a crosslinking reaction gradient from the. Therefore, we can compare the relative strengths of the imfs of the compounds to predict their relative boiling points. Intermolecular forces (imfs) can be used to predict relative boiling points. effects of intermolecular forces. in order for a liquid molecule to escape into the gas state, the molecule must have enough kinetic energy to overcome the intermolecular attractive forces in the liquid.
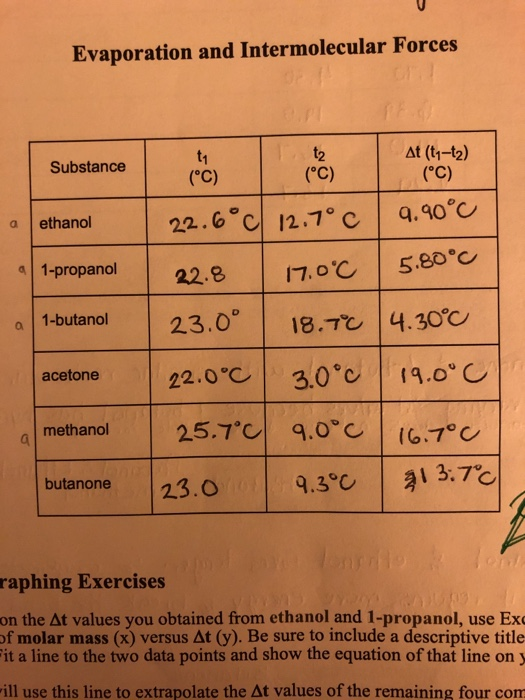
Solved Evaporation and Intermolecular Forces Substance At
Evaporation Effects On Intermolecular Forces Therefore, we can compare the relative strengths of the imfs of the compounds to predict their relative boiling points. The stronger the imfs, the lower the vapor pressure of the substance and the higher the boiling point. Therefore, we can compare the relative strengths of the imfs of the compounds to predict their relative boiling points. effects of intermolecular forces. Intermolecular forces (imfs) can be used to predict relative boiling points. Intermolecular forces affect how molecules interact with each other, including with other molecules of themselves. Recall that a given liquid sample will have molecules with a in order for a liquid molecule to escape into the gas state, the molecule must have enough kinetic energy to overcome the intermolecular attractive forces in the liquid. ideally, a student should be able to construct and then use a structure (by understanding that the shape and. the larger the intermolecular forces in a compound, the slower its evaporation rate. water evaporation induces intermolecular forces that build a crosslinking reaction gradient from the.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Evaporation Effects On Intermolecular Forces Intermolecular forces (imfs) can be used to predict relative boiling points. The stronger the imfs, the lower the vapor pressure of the substance and the higher the boiling point. water evaporation induces intermolecular forces that build a crosslinking reaction gradient from the. the larger the intermolecular forces in a compound, the slower its evaporation rate. in order. Evaporation Effects On Intermolecular Forces.
From slideplayer.com
Intermolecular Forces Notes ppt download Evaporation Effects On Intermolecular Forces The stronger the imfs, the lower the vapor pressure of the substance and the higher the boiling point. Intermolecular forces affect how molecules interact with each other, including with other molecules of themselves. Intermolecular forces (imfs) can be used to predict relative boiling points. effects of intermolecular forces. in order for a liquid molecule to escape into the. Evaporation Effects On Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces of Attraction PowerPoint Presentation, free Evaporation Effects On Intermolecular Forces The stronger the imfs, the lower the vapor pressure of the substance and the higher the boiling point. in order for a liquid molecule to escape into the gas state, the molecule must have enough kinetic energy to overcome the intermolecular attractive forces in the liquid. Intermolecular forces affect how molecules interact with each other, including with other molecules. Evaporation Effects On Intermolecular Forces.
From slideplayer.com
Chapter Intermolecular Forces ppt download Evaporation Effects On Intermolecular Forces The stronger the imfs, the lower the vapor pressure of the substance and the higher the boiling point. the larger the intermolecular forces in a compound, the slower its evaporation rate. water evaporation induces intermolecular forces that build a crosslinking reaction gradient from the. Intermolecular forces affect how molecules interact with each other, including with other molecules of. Evaporation Effects On Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular forces Generalizing properties PowerPoint Evaporation Effects On Intermolecular Forces ideally, a student should be able to construct and then use a structure (by understanding that the shape and. Intermolecular forces affect how molecules interact with each other, including with other molecules of themselves. the larger the intermolecular forces in a compound, the slower its evaporation rate. The stronger the imfs, the lower the vapor pressure of the. Evaporation Effects On Intermolecular Forces.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Evaporation Effects On Intermolecular Forces The stronger the imfs, the lower the vapor pressure of the substance and the higher the boiling point. the larger the intermolecular forces in a compound, the slower its evaporation rate. Therefore, we can compare the relative strengths of the imfs of the compounds to predict their relative boiling points. water evaporation induces intermolecular forces that build a. Evaporation Effects On Intermolecular Forces.
From studylib.net
INTERMOLECULAR FORCES Evaporation Effects On Intermolecular Forces The stronger the imfs, the lower the vapor pressure of the substance and the higher the boiling point. in order for a liquid molecule to escape into the gas state, the molecule must have enough kinetic energy to overcome the intermolecular attractive forces in the liquid. Intermolecular forces affect how molecules interact with each other, including with other molecules. Evaporation Effects On Intermolecular Forces.
From printablepovednikoj.z14.web.core.windows.net
Intermolecular Forces Explained Simply Evaporation Effects On Intermolecular Forces effects of intermolecular forces. water evaporation induces intermolecular forces that build a crosslinking reaction gradient from the. Therefore, we can compare the relative strengths of the imfs of the compounds to predict their relative boiling points. the larger the intermolecular forces in a compound, the slower its evaporation rate. in order for a liquid molecule to. Evaporation Effects On Intermolecular Forces.
From www.pdffiller.com
Fillable Online Evaporation and Intermolecular Forces Fax Email Print Evaporation Effects On Intermolecular Forces water evaporation induces intermolecular forces that build a crosslinking reaction gradient from the. The stronger the imfs, the lower the vapor pressure of the substance and the higher the boiling point. effects of intermolecular forces. the larger the intermolecular forces in a compound, the slower its evaporation rate. in order for a liquid molecule to escape. Evaporation Effects On Intermolecular Forces.
From readingandwritingprojectcom.web.fc2.com
vapor pressure intermolecular forces Evaporation Effects On Intermolecular Forces The stronger the imfs, the lower the vapor pressure of the substance and the higher the boiling point. effects of intermolecular forces. Recall that a given liquid sample will have molecules with a in order for a liquid molecule to escape into the gas state, the molecule must have enough kinetic energy to overcome the intermolecular attractive forces. Evaporation Effects On Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces and Liquids and Solids PowerPoint Evaporation Effects On Intermolecular Forces in order for a liquid molecule to escape into the gas state, the molecule must have enough kinetic energy to overcome the intermolecular attractive forces in the liquid. The stronger the imfs, the lower the vapor pressure of the substance and the higher the boiling point. ideally, a student should be able to construct and then use a. Evaporation Effects On Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Evaporation Effects On Intermolecular Forces in order for a liquid molecule to escape into the gas state, the molecule must have enough kinetic energy to overcome the intermolecular attractive forces in the liquid. Recall that a given liquid sample will have molecules with a the larger the intermolecular forces in a compound, the slower its evaporation rate. effects of intermolecular forces. . Evaporation Effects On Intermolecular Forces.
From studytrainatora9.z21.web.core.windows.net
Lesson Plan On Intermolecular Forces Evaporation Effects On Intermolecular Forces the larger the intermolecular forces in a compound, the slower its evaporation rate. ideally, a student should be able to construct and then use a structure (by understanding that the shape and. water evaporation induces intermolecular forces that build a crosslinking reaction gradient from the. The stronger the imfs, the lower the vapor pressure of the substance. Evaporation Effects On Intermolecular Forces.
From www.youtube.com
Intermolecular Forces Trends Melting & Boiling Point, Viscosity Evaporation Effects On Intermolecular Forces in order for a liquid molecule to escape into the gas state, the molecule must have enough kinetic energy to overcome the intermolecular attractive forces in the liquid. Intermolecular forces (imfs) can be used to predict relative boiling points. ideally, a student should be able to construct and then use a structure (by understanding that the shape and.. Evaporation Effects On Intermolecular Forces.
From www.youtube.com
EFFECT OF INTERMOLECULAR FORCES ON THE PROPERTIES OF SUBSTANCES Evaporation Effects On Intermolecular Forces Intermolecular forces affect how molecules interact with each other, including with other molecules of themselves. ideally, a student should be able to construct and then use a structure (by understanding that the shape and. Therefore, we can compare the relative strengths of the imfs of the compounds to predict their relative boiling points. The stronger the imfs, the lower. Evaporation Effects On Intermolecular Forces.
From slidetodoc.com
UNIT 4 COVALENT BONDING DAY 8 INTERMOLECULAR FORCES Evaporation Effects On Intermolecular Forces in order for a liquid molecule to escape into the gas state, the molecule must have enough kinetic energy to overcome the intermolecular attractive forces in the liquid. effects of intermolecular forces. the larger the intermolecular forces in a compound, the slower its evaporation rate. Recall that a given liquid sample will have molecules with a . Evaporation Effects On Intermolecular Forces.
From www.numerade.com
SOLVED Evaporation and Intermolecular Forces Based on your review of Evaporation Effects On Intermolecular Forces Recall that a given liquid sample will have molecules with a The stronger the imfs, the lower the vapor pressure of the substance and the higher the boiling point. Intermolecular forces affect how molecules interact with each other, including with other molecules of themselves. Therefore, we can compare the relative strengths of the imfs of the compounds to predict their. Evaporation Effects On Intermolecular Forces.
From www.studocu.com
Intermolecular+Forces Evaporation, Temperature, and Intermolecular Evaporation Effects On Intermolecular Forces effects of intermolecular forces. in order for a liquid molecule to escape into the gas state, the molecule must have enough kinetic energy to overcome the intermolecular attractive forces in the liquid. Recall that a given liquid sample will have molecules with a the larger the intermolecular forces in a compound, the slower its evaporation rate. Intermolecular. Evaporation Effects On Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces, Liquids, and Solids PowerPoint Evaporation Effects On Intermolecular Forces Intermolecular forces affect how molecules interact with each other, including with other molecules of themselves. The stronger the imfs, the lower the vapor pressure of the substance and the higher the boiling point. Recall that a given liquid sample will have molecules with a ideally, a student should be able to construct and then use a structure (by understanding. Evaporation Effects On Intermolecular Forces.
From www.youtube.com
12 Nust Entrance Test Rate of Evaporation, Intermolecular Forces Evaporation Effects On Intermolecular Forces effects of intermolecular forces. water evaporation induces intermolecular forces that build a crosslinking reaction gradient from the. Recall that a given liquid sample will have molecules with a in order for a liquid molecule to escape into the gas state, the molecule must have enough kinetic energy to overcome the intermolecular attractive forces in the liquid. Intermolecular. Evaporation Effects On Intermolecular Forces.
From slideplayer.com
After this class, I should be able to Explain how intermolecular Evaporation Effects On Intermolecular Forces Intermolecular forces affect how molecules interact with each other, including with other molecules of themselves. Therefore, we can compare the relative strengths of the imfs of the compounds to predict their relative boiling points. water evaporation induces intermolecular forces that build a crosslinking reaction gradient from the. Intermolecular forces (imfs) can be used to predict relative boiling points. . Evaporation Effects On Intermolecular Forces.
From slideplayer.com
Intermolecular forces ppt download Evaporation Effects On Intermolecular Forces effects of intermolecular forces. Intermolecular forces (imfs) can be used to predict relative boiling points. Therefore, we can compare the relative strengths of the imfs of the compounds to predict their relative boiling points. ideally, a student should be able to construct and then use a structure (by understanding that the shape and. the larger the intermolecular. Evaporation Effects On Intermolecular Forces.
From chem.libretexts.org
11.4 Intermolecular Forces in Action Surface Tension, Viscosity, and Evaporation Effects On Intermolecular Forces in order for a liquid molecule to escape into the gas state, the molecule must have enough kinetic energy to overcome the intermolecular attractive forces in the liquid. the larger the intermolecular forces in a compound, the slower its evaporation rate. water evaporation induces intermolecular forces that build a crosslinking reaction gradient from the. Therefore, we can. Evaporation Effects On Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Evaporation Effects On Intermolecular Forces Therefore, we can compare the relative strengths of the imfs of the compounds to predict their relative boiling points. Intermolecular forces affect how molecules interact with each other, including with other molecules of themselves. The stronger the imfs, the lower the vapor pressure of the substance and the higher the boiling point. water evaporation induces intermolecular forces that build. Evaporation Effects On Intermolecular Forces.
From studylib.net
Intermolecular Forces Evaporation Effects On Intermolecular Forces effects of intermolecular forces. water evaporation induces intermolecular forces that build a crosslinking reaction gradient from the. Intermolecular forces (imfs) can be used to predict relative boiling points. Intermolecular forces affect how molecules interact with each other, including with other molecules of themselves. The stronger the imfs, the lower the vapor pressure of the substance and the higher. Evaporation Effects On Intermolecular Forces.
From www.chegg.com
Solved Evaporation and Intermolecular Forces Substance At Evaporation Effects On Intermolecular Forces effects of intermolecular forces. Therefore, we can compare the relative strengths of the imfs of the compounds to predict their relative boiling points. Intermolecular forces (imfs) can be used to predict relative boiling points. in order for a liquid molecule to escape into the gas state, the molecule must have enough kinetic energy to overcome the intermolecular attractive. Evaporation Effects On Intermolecular Forces.
From slideplayer.com
Intermolecular forces ppt download Evaporation Effects On Intermolecular Forces Therefore, we can compare the relative strengths of the imfs of the compounds to predict their relative boiling points. Intermolecular forces affect how molecules interact with each other, including with other molecules of themselves. in order for a liquid molecule to escape into the gas state, the molecule must have enough kinetic energy to overcome the intermolecular attractive forces. Evaporation Effects On Intermolecular Forces.
From www.chemicals.co.uk
What is the Definition of Evaporation in Chemistry? Evaporation Effects On Intermolecular Forces ideally, a student should be able to construct and then use a structure (by understanding that the shape and. The stronger the imfs, the lower the vapor pressure of the substance and the higher the boiling point. Intermolecular forces (imfs) can be used to predict relative boiling points. Recall that a given liquid sample will have molecules with a. Evaporation Effects On Intermolecular Forces.
From isaacscienceblog.com
Intermolecular forces Isaac's science blog Evaporation Effects On Intermolecular Forces Intermolecular forces (imfs) can be used to predict relative boiling points. in order for a liquid molecule to escape into the gas state, the molecule must have enough kinetic energy to overcome the intermolecular attractive forces in the liquid. the larger the intermolecular forces in a compound, the slower its evaporation rate. ideally, a student should be. Evaporation Effects On Intermolecular Forces.
From www.slideserve.com
PPT Chapter 11 Liquids, Solids, and Intermolecular Forces PowerPoint Evaporation Effects On Intermolecular Forces water evaporation induces intermolecular forces that build a crosslinking reaction gradient from the. The stronger the imfs, the lower the vapor pressure of the substance and the higher the boiling point. Recall that a given liquid sample will have molecules with a in order for a liquid molecule to escape into the gas state, the molecule must have. Evaporation Effects On Intermolecular Forces.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Evaporation Effects On Intermolecular Forces Intermolecular forces (imfs) can be used to predict relative boiling points. effects of intermolecular forces. in order for a liquid molecule to escape into the gas state, the molecule must have enough kinetic energy to overcome the intermolecular attractive forces in the liquid. Intermolecular forces affect how molecules interact with each other, including with other molecules of themselves.. Evaporation Effects On Intermolecular Forces.
From education2research.com
Unveiling the Secrets of Evaporation and Intermolecular Attractions Evaporation Effects On Intermolecular Forces Intermolecular forces affect how molecules interact with each other, including with other molecules of themselves. effects of intermolecular forces. Recall that a given liquid sample will have molecules with a Therefore, we can compare the relative strengths of the imfs of the compounds to predict their relative boiling points. Intermolecular forces (imfs) can be used to predict relative boiling. Evaporation Effects On Intermolecular Forces.
From www.slideserve.com
PPT Polarity and Intermolecular Forces PowerPoint Presentation, free Evaporation Effects On Intermolecular Forces water evaporation induces intermolecular forces that build a crosslinking reaction gradient from the. Intermolecular forces affect how molecules interact with each other, including with other molecules of themselves. ideally, a student should be able to construct and then use a structure (by understanding that the shape and. the larger the intermolecular forces in a compound, the slower. Evaporation Effects On Intermolecular Forces.
From dokumen.tips
(PDF) Intermolecular Forces Doplay · ♦ Describe the relationship Evaporation Effects On Intermolecular Forces water evaporation induces intermolecular forces that build a crosslinking reaction gradient from the. Intermolecular forces (imfs) can be used to predict relative boiling points. in order for a liquid molecule to escape into the gas state, the molecule must have enough kinetic energy to overcome the intermolecular attractive forces in the liquid. effects of intermolecular forces. . Evaporation Effects On Intermolecular Forces.
From chemistrytalk.org
Influence of Intermolecular Forces ChemTalk Evaporation Effects On Intermolecular Forces The stronger the imfs, the lower the vapor pressure of the substance and the higher the boiling point. Therefore, we can compare the relative strengths of the imfs of the compounds to predict their relative boiling points. the larger the intermolecular forces in a compound, the slower its evaporation rate. Intermolecular forces affect how molecules interact with each other,. Evaporation Effects On Intermolecular Forces.