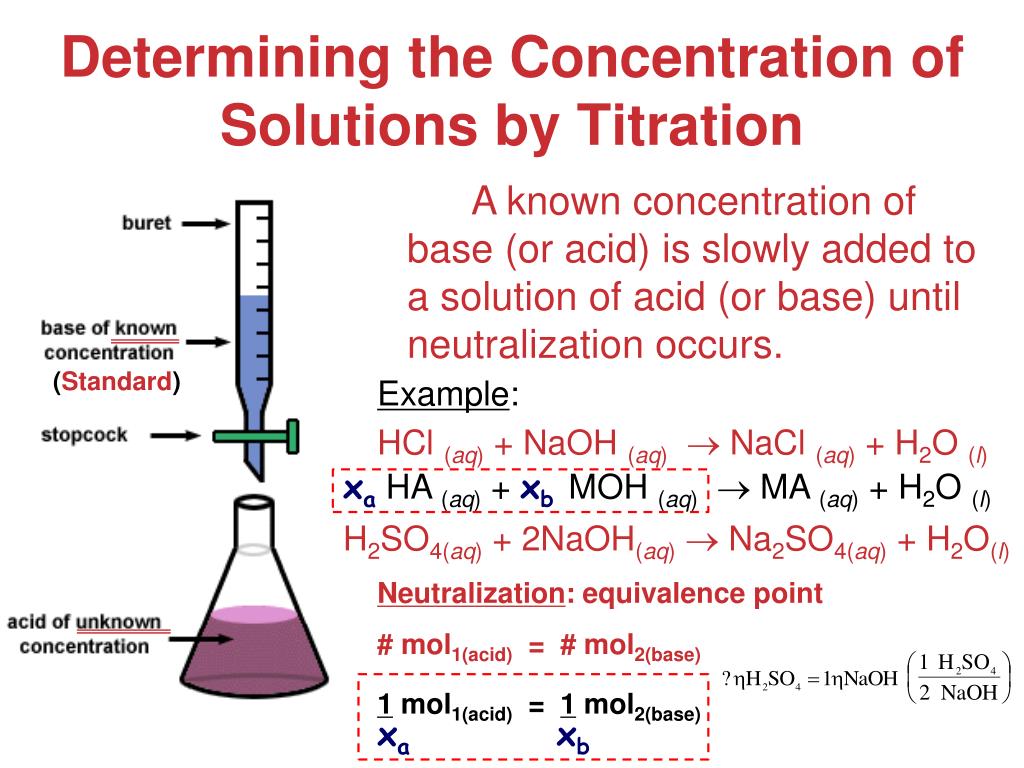
How To Do Titration Reactions . Describe how to perform a titration experiment. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Perform calculations to determine concentration of unknown acid or base. The next step is to exactly react it with something that it reacts with. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. The first step is to measure out a known volume of the sample that you want to find the concentration of into a flask. The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance. In our example we would accurately measure a known volume of the hydrochloric acid solution into the flask. First determine the moles of \(\ce{naoh}\) in the reaction.
from www.slideserve.com
Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. First determine the moles of \(\ce{naoh}\) in the reaction. Describe how to perform a titration experiment. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed. The first step is to measure out a known volume of the sample that you want to find the concentration of into a flask. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The next step is to exactly react it with something that it reacts with. The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance. Perform calculations to determine concentration of unknown acid or base. In our example we would accurately measure a known volume of the hydrochloric acid solution into the flask.
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation
How To Do Titration Reactions Perform calculations to determine concentration of unknown acid or base. First determine the moles of \(\ce{naoh}\) in the reaction. The next step is to exactly react it with something that it reacts with. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Describe how to perform a titration experiment. Perform calculations to determine concentration of unknown acid or base. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed. The first step is to measure out a known volume of the sample that you want to find the concentration of into a flask. The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In our example we would accurately measure a known volume of the hydrochloric acid solution into the flask.
From www.youtube.com
How to perform titration experiment class 12 oxalic acid? YouTube How To Do Titration Reactions From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed. The purpose of any titration is to determine the amount of analyte present by reacting away every last. How To Do Titration Reactions.
From learningschoolkdebra61.z14.web.core.windows.net
How To Do Titrations In Chemistry How To Do Titration Reactions Perform calculations to determine concentration of unknown acid or base. The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance. Describe how to perform a titration experiment. In our example we would accurately measure a known volume of the hydrochloric acid solution into the flask. A titration is. How To Do Titration Reactions.
From www.youtube.com
Titration calculation example Chemistry Khan Academy YouTube How To Do Titration Reactions A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The next step is to exactly react it with something that it reacts with. Describe how to perform a titration experiment. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. In our example we would accurately measure a. How To Do Titration Reactions.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog How To Do Titration Reactions In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Describe how to perform a titration experiment. The first step is to measure out a known volume of. How To Do Titration Reactions.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube How To Do Titration Reactions In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed. The next step is to exactly react it with something that it reacts with. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Titration is the slow. How To Do Titration Reactions.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations How To Do Titration Reactions In our example we would accurately measure a known volume of the hydrochloric acid solution into the flask. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed. The first step is to measure out a known volume of. How To Do Titration Reactions.
From present5.com
Titration and AcidBase Neutralization LEARNING OBJECTIVES 11.2.2.7 How To Do Titration Reactions A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance. In our example we would accurately measure. How To Do Titration Reactions.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube How To Do Titration Reactions Perform calculations to determine concentration of unknown acid or base. First determine the moles of \(\ce{naoh}\) in the reaction. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Describe how to perform a titration. How To Do Titration Reactions.
From www.youtube.com
How To Do Titrations Chemical Calculations Chemistry FuseSchool How To Do Titration Reactions Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance. The next step is to exactly react it with something that it reacts with.. How To Do Titration Reactions.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint How To Do Titration Reactions From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. In our example we would accurately measure a known volume of the hydrochloric acid solution into the flask. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The first step is to measure out. How To Do Titration Reactions.
From www.slideserve.com
PPT Ch 16 Redox Titrations PowerPoint Presentation ID6602548 How To Do Titration Reactions In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed. Perform calculations to determine concentration of unknown acid or base. The purpose of any titration is to determine the amount of analyte present by reacting away every last bit. How To Do Titration Reactions.
From theedge.com.hk
Chemistry How To Titration The Edge How To Do Titration Reactions The first step is to measure out a known volume of the sample that you want to find the concentration of into a flask. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. First determine the moles of \(\ce{naoh}\) in the reaction. Describe how to. How To Do Titration Reactions.
From www.youtube.com
WCLN Titrations Involving Precipitation Reactions Chemistry YouTube How To Do Titration Reactions Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. In our example we would accurately measure a known volume of the hydrochloric acid solution into the flask. Perform calculations to determine concentration of unknown acid or base. First determine the moles of \(\ce{naoh}\) in the. How To Do Titration Reactions.
From www.youtube.com
Solving AcidBase Titration Problems YouTube How To Do Titration Reactions In our example we would accurately measure a known volume of the hydrochloric acid solution into the flask. The first step is to measure out a known volume of the sample that you want to find the concentration of into a flask. The next step is to exactly react it with something that it reacts with. Titration is the slow. How To Do Titration Reactions.
From studyespacioec4o.z22.web.core.windows.net
How To Do Titrations In Chemistry How To Do Titration Reactions From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance. First determine the. How To Do Titration Reactions.
From www.youtube.com
Chemistry How to do Titrations for Paper 3 // 9701/33/O/N/22 YouTube How To Do Titration Reactions The next step is to exactly react it with something that it reacts with. The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance. First determine the moles of \(\ce{naoh}\) in the reaction. In our example we would accurately measure a known volume of the hydrochloric acid solution. How To Do Titration Reactions.
From letitsnowglobe.co.uk
Titration procedure pdf How To Do Titration Reactions In our example we would accurately measure a known volume of the hydrochloric acid solution into the flask. The next step is to exactly react it with something that it reacts with. First determine the moles of \(\ce{naoh}\) in the reaction. Describe how to perform a titration experiment. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. The. How To Do Titration Reactions.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation How To Do Titration Reactions In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed. In our example we would accurately measure a known volume of the hydrochloric acid solution into the flask. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted.. How To Do Titration Reactions.
From www.youtube.com
Acids and Bases, Titrations, and Neutralization Reactions YouTube How To Do Titration Reactions Perform calculations to determine concentration of unknown acid or base. The first step is to measure out a known volume of the sample that you want to find the concentration of into a flask. The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance. In this tutorial, you. How To Do Titration Reactions.
From www.tes.com
Titration Edexcel 91 Separate (Triple) Science Teaching Resources How To Do Titration Reactions In our example we would accurately measure a known volume of the hydrochloric acid solution into the flask. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution. How To Do Titration Reactions.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators How To Do Titration Reactions From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. The next step is to exactly react it with something that it reacts with. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. First determine the moles of \(\ce{naoh}\) in the reaction. Perform calculations. How To Do Titration Reactions.
From www.youtube.com
REDOX REACTIONS AS THE BASIS FOR TITRATIONS YouTube How To Do Titration Reactions Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The next step is to exactly react it with something that it reacts with. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make. How To Do Titration Reactions.
From chem4three.blogspot.ca
CHEMISTRY 11 TITRATIONS How To Do Titration Reactions Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. First determine the moles of \(\ce{naoh}\) in the reaction. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Perform calculations to determine concentration of unknown. How To Do Titration Reactions.
From www.studocu.com
CHEM272 How to do Experiment 7 Notes Back Titration Review Of Redox How To Do Titration Reactions The first step is to measure out a known volume of the sample that you want to find the concentration of into a flask. Describe how to perform a titration experiment. First determine the moles of \(\ce{naoh}\) in the reaction. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.. How To Do Titration Reactions.
From www.sliderbase.com
Titration How To Do Titration Reactions Describe how to perform a titration experiment. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration is performed. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution. How To Do Titration Reactions.
From www.slideserve.com
PPT ENZYME CATALYSIS LAB PowerPoint Presentation, free download ID How To Do Titration Reactions Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. First determine the moles of \(\ce{naoh}\) in the reaction. The first step is to measure out a known volume of the sample that you want. How To Do Titration Reactions.
From www.youtube.com
5 Solving Titration problems with acids and bases YouTube How To Do Titration Reactions A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as well as how titration. How To Do Titration Reactions.
From www.ck12.org
Titration (Calculations) Example 2 ( Video ) Chemistry CK12 How To Do Titration Reactions Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Describe how to perform a titration experiment. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The purpose of any titration is to determine the. How To Do Titration Reactions.
From www.science-revision.co.uk
Titrations How To Do Titration Reactions Describe how to perform a titration experiment. The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance. In our example we would accurately measure a known volume of the hydrochloric acid solution into the flask. A titration is a laboratory technique used to precisely measure molar concentration of. How To Do Titration Reactions.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation How To Do Titration Reactions Perform calculations to determine concentration of unknown acid or base. The next step is to exactly react it with something that it reacts with. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. The purpose of any titration is to determine the amount of analyte present by reacting away every last bit of that substance. A titration is. How To Do Titration Reactions.
From www.tutormyself.com
(f) Acids, alkalis and titrations Archives TutorMyself Chemistry How To Do Titration Reactions Describe how to perform a titration experiment. The next step is to exactly react it with something that it reacts with. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The purpose of any titration is to determine the amount of analyte present by reacting. How To Do Titration Reactions.
From printablehaferbrotwp.z21.web.core.windows.net
How To Do Titrations In Chemistry How To Do Titration Reactions In our example we would accurately measure a known volume of the hydrochloric acid solution into the flask. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Describe how to perform a titration experiment. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work,. How To Do Titration Reactions.
From www.microlit.com
An Advanced Guide to Titration Microlit How To Do Titration Reactions A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Describe how to perform a. How To Do Titration Reactions.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 How To Do Titration Reactions From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Perform calculations to determine concentration of unknown acid or base. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume. How To Do Titration Reactions.
From www.showme.com
Titration calculations Science, Chemistry, Chemicalreactions How To Do Titration Reactions From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Describe how to perform a titration experiment. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The first step is to measure out a known volume of the sample that you want to find. How To Do Titration Reactions.