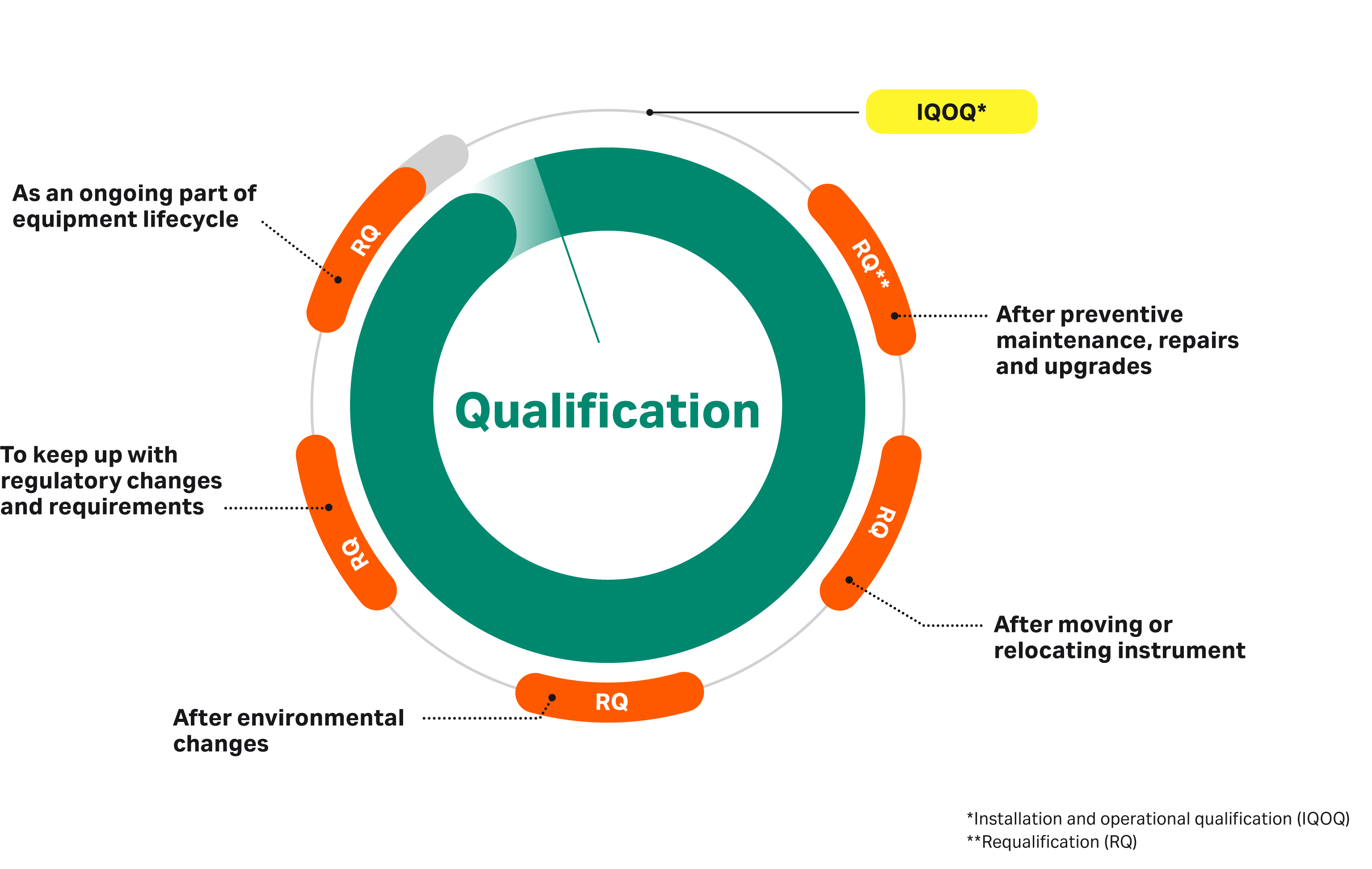
Qualification Of Laboratory Instrumentation Validation And Transfer . For a bla, as discussed, you must obtain prior approval from fda before implementing a change in analytical methods if those methods are specified in fda. Method validation (or qualification) should follow good manufacturing practice (gmp) requirements to prove that a method is fit for. If you traded in the old system or it is not functional, run qc samples on the new. Select at least one validation or verification study. Table 1 provides a list. Follow procedures in sm xx20 b or epa method qc section. Learn how to ensure that the laboratory equipment you use is qualified for its intended use, how to ensure regulatory compliance, how to document. How does your laboratory validate clinical claims made by the laboratory about ldts?
from www.cytivalifesciences.com
Follow procedures in sm xx20 b or epa method qc section. Table 1 provides a list. If you traded in the old system or it is not functional, run qc samples on the new. For a bla, as discussed, you must obtain prior approval from fda before implementing a change in analytical methods if those methods are specified in fda. Learn how to ensure that the laboratory equipment you use is qualified for its intended use, how to ensure regulatory compliance, how to document. Select at least one validation or verification study. Method validation (or qualification) should follow good manufacturing practice (gmp) requirements to prove that a method is fit for. How does your laboratory validate clinical claims made by the laboratory about ldts?
Stay compliant instrument qualification for cGMP Cytiva
Qualification Of Laboratory Instrumentation Validation And Transfer If you traded in the old system or it is not functional, run qc samples on the new. How does your laboratory validate clinical claims made by the laboratory about ldts? Follow procedures in sm xx20 b or epa method qc section. Method validation (or qualification) should follow good manufacturing practice (gmp) requirements to prove that a method is fit for. Table 1 provides a list. Learn how to ensure that the laboratory equipment you use is qualified for its intended use, how to ensure regulatory compliance, how to document. Select at least one validation or verification study. If you traded in the old system or it is not functional, run qc samples on the new. For a bla, as discussed, you must obtain prior approval from fda before implementing a change in analytical methods if those methods are specified in fda.
From www.presentationeze.com
Equipment Validation Facility Qualification Material Qualification Of Laboratory Instrumentation Validation And Transfer Method validation (or qualification) should follow good manufacturing practice (gmp) requirements to prove that a method is fit for. Select at least one validation or verification study. For a bla, as discussed, you must obtain prior approval from fda before implementing a change in analytical methods if those methods are specified in fda. Learn how to ensure that the laboratory. Qualification Of Laboratory Instrumentation Validation And Transfer.
From www.learngxp.com
Equipment Qualification for Analytical Laboratory Instruments Qualification Of Laboratory Instrumentation Validation And Transfer Method validation (or qualification) should follow good manufacturing practice (gmp) requirements to prove that a method is fit for. For a bla, as discussed, you must obtain prior approval from fda before implementing a change in analytical methods if those methods are specified in fda. Learn how to ensure that the laboratory equipment you use is qualified for its intended. Qualification Of Laboratory Instrumentation Validation And Transfer.
From www.kewaunee.in
Understanding Lab Validation and Qualification Kewaunee Qualification Of Laboratory Instrumentation Validation And Transfer If you traded in the old system or it is not functional, run qc samples on the new. For a bla, as discussed, you must obtain prior approval from fda before implementing a change in analytical methods if those methods are specified in fda. Learn how to ensure that the laboratory equipment you use is qualified for its intended use,. Qualification Of Laboratory Instrumentation Validation And Transfer.
From www.cytivalifesciences.com
Stay compliant instrument qualification for cGMP Cytiva Qualification Of Laboratory Instrumentation Validation And Transfer If you traded in the old system or it is not functional, run qc samples on the new. For a bla, as discussed, you must obtain prior approval from fda before implementing a change in analytical methods if those methods are specified in fda. Learn how to ensure that the laboratory equipment you use is qualified for its intended use,. Qualification Of Laboratory Instrumentation Validation And Transfer.
From www.sandia.gov
Instrumentation Validation and Qualification Sciences Experimental Qualification Of Laboratory Instrumentation Validation And Transfer Select at least one validation or verification study. Learn how to ensure that the laboratory equipment you use is qualified for its intended use, how to ensure regulatory compliance, how to document. Method validation (or qualification) should follow good manufacturing practice (gmp) requirements to prove that a method is fit for. If you traded in the old system or it. Qualification Of Laboratory Instrumentation Validation And Transfer.
From present5.com
Current Validation Process and Issues Exemplified by the Qualification Of Laboratory Instrumentation Validation And Transfer Select at least one validation or verification study. How does your laboratory validate clinical claims made by the laboratory about ldts? For a bla, as discussed, you must obtain prior approval from fda before implementing a change in analytical methods if those methods are specified in fda. Follow procedures in sm xx20 b or epa method qc section. If you. Qualification Of Laboratory Instrumentation Validation And Transfer.
From www.presentationeze.com
Stages to equipment validation PresentationEZE Qualification Of Laboratory Instrumentation Validation And Transfer Follow procedures in sm xx20 b or epa method qc section. Learn how to ensure that the laboratory equipment you use is qualified for its intended use, how to ensure regulatory compliance, how to document. Method validation (or qualification) should follow good manufacturing practice (gmp) requirements to prove that a method is fit for. For a bla, as discussed, you. Qualification Of Laboratory Instrumentation Validation And Transfer.
From www.researchgate.net
(PDF) Validation, Qualification and the GMP Laboratory Qualification Of Laboratory Instrumentation Validation And Transfer Select at least one validation or verification study. Learn how to ensure that the laboratory equipment you use is qualified for its intended use, how to ensure regulatory compliance, how to document. How does your laboratory validate clinical claims made by the laboratory about ldts? Follow procedures in sm xx20 b or epa method qc section. Method validation (or qualification). Qualification Of Laboratory Instrumentation Validation And Transfer.
From www.semanticscholar.org
AN OVERVIEW OF ANALYTICAL INSTRUMENT QUALIFICATION WITH REFERENCE OF Qualification Of Laboratory Instrumentation Validation And Transfer How does your laboratory validate clinical claims made by the laboratory about ldts? Select at least one validation or verification study. Table 1 provides a list. Learn how to ensure that the laboratory equipment you use is qualified for its intended use, how to ensure regulatory compliance, how to document. Follow procedures in sm xx20 b or epa method qc. Qualification Of Laboratory Instrumentation Validation And Transfer.
From www.kewaunee.in
HVAC Validation and Qualification for Laboratories Kewaunee Qualification Of Laboratory Instrumentation Validation And Transfer Learn how to ensure that the laboratory equipment you use is qualified for its intended use, how to ensure regulatory compliance, how to document. How does your laboratory validate clinical claims made by the laboratory about ldts? If you traded in the old system or it is not functional, run qc samples on the new. For a bla, as discussed,. Qualification Of Laboratory Instrumentation Validation And Transfer.
From cerf-notebook.com
IQ, OQ Validation CERF Electronic Lab Notebook Qualification Of Laboratory Instrumentation Validation And Transfer Select at least one validation or verification study. Follow procedures in sm xx20 b or epa method qc section. Table 1 provides a list. Method validation (or qualification) should follow good manufacturing practice (gmp) requirements to prove that a method is fit for. For a bla, as discussed, you must obtain prior approval from fda before implementing a change in. Qualification Of Laboratory Instrumentation Validation And Transfer.
From present5.com
Current Validation Process and Issues Exemplified by the Qualification Of Laboratory Instrumentation Validation And Transfer How does your laboratory validate clinical claims made by the laboratory about ldts? For a bla, as discussed, you must obtain prior approval from fda before implementing a change in analytical methods if those methods are specified in fda. Table 1 provides a list. If you traded in the old system or it is not functional, run qc samples on. Qualification Of Laboratory Instrumentation Validation And Transfer.
From www.slideserve.com
PPT 3. Validation (and Qualification) PowerPoint Presentation, free Qualification Of Laboratory Instrumentation Validation And Transfer Follow procedures in sm xx20 b or epa method qc section. If you traded in the old system or it is not functional, run qc samples on the new. How does your laboratory validate clinical claims made by the laboratory about ldts? Select at least one validation or verification study. Table 1 provides a list. Learn how to ensure that. Qualification Of Laboratory Instrumentation Validation And Transfer.
From www.yumpu.com
Analytical Instrument Qualification and System Validation Qualification Of Laboratory Instrumentation Validation And Transfer Method validation (or qualification) should follow good manufacturing practice (gmp) requirements to prove that a method is fit for. Learn how to ensure that the laboratory equipment you use is qualified for its intended use, how to ensure regulatory compliance, how to document. How does your laboratory validate clinical claims made by the laboratory about ldts? For a bla, as. Qualification Of Laboratory Instrumentation Validation And Transfer.
From www.slideshare.net
Validation Master Plan Qualification Of Laboratory Instrumentation Validation And Transfer Method validation (or qualification) should follow good manufacturing practice (gmp) requirements to prove that a method is fit for. If you traded in the old system or it is not functional, run qc samples on the new. Table 1 provides a list. Learn how to ensure that the laboratory equipment you use is qualified for its intended use, how to. Qualification Of Laboratory Instrumentation Validation And Transfer.
From www.getreskilled.com
Equipment Qualification Protocol Step by Step Writing Guide Qualification Of Laboratory Instrumentation Validation And Transfer If you traded in the old system or it is not functional, run qc samples on the new. How does your laboratory validate clinical claims made by the laboratory about ldts? Select at least one validation or verification study. Table 1 provides a list. For a bla, as discussed, you must obtain prior approval from fda before implementing a change. Qualification Of Laboratory Instrumentation Validation And Transfer.
From www.slideserve.com
PPT Validation and Qualification Overview PowerPoint Presentation Qualification Of Laboratory Instrumentation Validation And Transfer Learn how to ensure that the laboratory equipment you use is qualified for its intended use, how to ensure regulatory compliance, how to document. Table 1 provides a list. Follow procedures in sm xx20 b or epa method qc section. Select at least one validation or verification study. If you traded in the old system or it is not functional,. Qualification Of Laboratory Instrumentation Validation And Transfer.
From www.drugfuture.com
ANALYTICAL INSTRUMENT QUALIFICATION Qualification Of Laboratory Instrumentation Validation And Transfer How does your laboratory validate clinical claims made by the laboratory about ldts? For a bla, as discussed, you must obtain prior approval from fda before implementing a change in analytical methods if those methods are specified in fda. If you traded in the old system or it is not functional, run qc samples on the new. Follow procedures in. Qualification Of Laboratory Instrumentation Validation And Transfer.
From www.scribd.com
Qualification of GC & GC/MS Systems Laboratory Instrument Qualification Of Laboratory Instrumentation Validation And Transfer For a bla, as discussed, you must obtain prior approval from fda before implementing a change in analytical methods if those methods are specified in fda. How does your laboratory validate clinical claims made by the laboratory about ldts? If you traded in the old system or it is not functional, run qc samples on the new. Select at least. Qualification Of Laboratory Instrumentation Validation And Transfer.
From www.slideserve.com
PPT Qualification and Validation PowerPoint Presentation, free Qualification Of Laboratory Instrumentation Validation And Transfer Method validation (or qualification) should follow good manufacturing practice (gmp) requirements to prove that a method is fit for. How does your laboratory validate clinical claims made by the laboratory about ldts? Follow procedures in sm xx20 b or epa method qc section. If you traded in the old system or it is not functional, run qc samples on the. Qualification Of Laboratory Instrumentation Validation And Transfer.
From www.semanticscholar.org
Figure 3 from Overview of Risk‐Based Approach to Phase Appropriate Qualification Of Laboratory Instrumentation Validation And Transfer Method validation (or qualification) should follow good manufacturing practice (gmp) requirements to prove that a method is fit for. Learn how to ensure that the laboratory equipment you use is qualified for its intended use, how to ensure regulatory compliance, how to document. Select at least one validation or verification study. How does your laboratory validate clinical claims made by. Qualification Of Laboratory Instrumentation Validation And Transfer.
From gahess.com
Best Practices for Instrument Validation and Qualification (2022) Qualification Of Laboratory Instrumentation Validation And Transfer Follow procedures in sm xx20 b or epa method qc section. Learn how to ensure that the laboratory equipment you use is qualified for its intended use, how to ensure regulatory compliance, how to document. Select at least one validation or verification study. If you traded in the old system or it is not functional, run qc samples on the. Qualification Of Laboratory Instrumentation Validation And Transfer.
From www.youtube.com
HPLC Instrument Qualification and Method Validation YouTube Qualification Of Laboratory Instrumentation Validation And Transfer For a bla, as discussed, you must obtain prior approval from fda before implementing a change in analytical methods if those methods are specified in fda. Method validation (or qualification) should follow good manufacturing practice (gmp) requirements to prove that a method is fit for. How does your laboratory validate clinical claims made by the laboratory about ldts? If you. Qualification Of Laboratory Instrumentation Validation And Transfer.
From www.azom.com
Analytical Method Validation in Thermal Analysis Qualification Of Laboratory Instrumentation Validation And Transfer Learn how to ensure that the laboratory equipment you use is qualified for its intended use, how to ensure regulatory compliance, how to document. Follow procedures in sm xx20 b or epa method qc section. Table 1 provides a list. Method validation (or qualification) should follow good manufacturing practice (gmp) requirements to prove that a method is fit for. For. Qualification Of Laboratory Instrumentation Validation And Transfer.
From mpl.loesungsfabrik.de
Calibration, qualification and validation in the lab what's that? Qualification Of Laboratory Instrumentation Validation And Transfer Learn how to ensure that the laboratory equipment you use is qualified for its intended use, how to ensure regulatory compliance, how to document. Table 1 provides a list. Method validation (or qualification) should follow good manufacturing practice (gmp) requirements to prove that a method is fit for. If you traded in the old system or it is not functional,. Qualification Of Laboratory Instrumentation Validation And Transfer.
From www.researchgate.net
Template of a validation certificate. Download Scientific Diagram Qualification Of Laboratory Instrumentation Validation And Transfer Learn how to ensure that the laboratory equipment you use is qualified for its intended use, how to ensure regulatory compliance, how to document. Follow procedures in sm xx20 b or epa method qc section. How does your laboratory validate clinical claims made by the laboratory about ldts? Table 1 provides a list. Select at least one validation or verification. Qualification Of Laboratory Instrumentation Validation And Transfer.
From www.rotronic.com
Validation / Qualification Rotronic USA Qualification Of Laboratory Instrumentation Validation And Transfer Follow procedures in sm xx20 b or epa method qc section. Method validation (or qualification) should follow good manufacturing practice (gmp) requirements to prove that a method is fit for. How does your laboratory validate clinical claims made by the laboratory about ldts? Table 1 provides a list. Select at least one validation or verification study. Learn how to ensure. Qualification Of Laboratory Instrumentation Validation And Transfer.
From www.slideserve.com
PPT 3. Validation (and Qualification) PowerPoint Presentation, free Qualification Of Laboratory Instrumentation Validation And Transfer Table 1 provides a list. How does your laboratory validate clinical claims made by the laboratory about ldts? Learn how to ensure that the laboratory equipment you use is qualified for its intended use, how to ensure regulatory compliance, how to document. For a bla, as discussed, you must obtain prior approval from fda before implementing a change in analytical. Qualification Of Laboratory Instrumentation Validation And Transfer.
From www.slideserve.com
PPT 3. Validation (and Qualification) PowerPoint Presentation, free Qualification Of Laboratory Instrumentation Validation And Transfer Method validation (or qualification) should follow good manufacturing practice (gmp) requirements to prove that a method is fit for. If you traded in the old system or it is not functional, run qc samples on the new. Select at least one validation or verification study. For a bla, as discussed, you must obtain prior approval from fda before implementing a. Qualification Of Laboratory Instrumentation Validation And Transfer.
From www.careofair.com
Laboratory equipment qualification and validation Qualification Of Laboratory Instrumentation Validation And Transfer Learn how to ensure that the laboratory equipment you use is qualified for its intended use, how to ensure regulatory compliance, how to document. Select at least one validation or verification study. Follow procedures in sm xx20 b or epa method qc section. Table 1 provides a list. How does your laboratory validate clinical claims made by the laboratory about. Qualification Of Laboratory Instrumentation Validation And Transfer.
From www.slideserve.com
PPT Qualification and Validation PowerPoint Presentation, free Qualification Of Laboratory Instrumentation Validation And Transfer For a bla, as discussed, you must obtain prior approval from fda before implementing a change in analytical methods if those methods are specified in fda. Follow procedures in sm xx20 b or epa method qc section. Method validation (or qualification) should follow good manufacturing practice (gmp) requirements to prove that a method is fit for. How does your laboratory. Qualification Of Laboratory Instrumentation Validation And Transfer.
From slideplayer.com
Good Laboratory Practices ppt download Qualification Of Laboratory Instrumentation Validation And Transfer Select at least one validation or verification study. How does your laboratory validate clinical claims made by the laboratory about ldts? Method validation (or qualification) should follow good manufacturing practice (gmp) requirements to prove that a method is fit for. Learn how to ensure that the laboratory equipment you use is qualified for its intended use, how to ensure regulatory. Qualification Of Laboratory Instrumentation Validation And Transfer.
From www.slideserve.com
PPT VALIDATION METHODOLOGY PowerPoint Presentation, free download Qualification Of Laboratory Instrumentation Validation And Transfer If you traded in the old system or it is not functional, run qc samples on the new. For a bla, as discussed, you must obtain prior approval from fda before implementing a change in analytical methods if those methods are specified in fda. How does your laboratory validate clinical claims made by the laboratory about ldts? Follow procedures in. Qualification Of Laboratory Instrumentation Validation And Transfer.
From kvalito.ch
Commissioning, Qualification, and Validation (CQV) for Facility Qualification Of Laboratory Instrumentation Validation And Transfer Follow procedures in sm xx20 b or epa method qc section. Learn how to ensure that the laboratory equipment you use is qualified for its intended use, how to ensure regulatory compliance, how to document. Method validation (or qualification) should follow good manufacturing practice (gmp) requirements to prove that a method is fit for. Select at least one validation or. Qualification Of Laboratory Instrumentation Validation And Transfer.
From slidetodoc.com
Qualification And Validation In Pharmaceutical Manufacturing KHADIJAH Qualification Of Laboratory Instrumentation Validation And Transfer How does your laboratory validate clinical claims made by the laboratory about ldts? Table 1 provides a list. Method validation (or qualification) should follow good manufacturing practice (gmp) requirements to prove that a method is fit for. Follow procedures in sm xx20 b or epa method qc section. Learn how to ensure that the laboratory equipment you use is qualified. Qualification Of Laboratory Instrumentation Validation And Transfer.