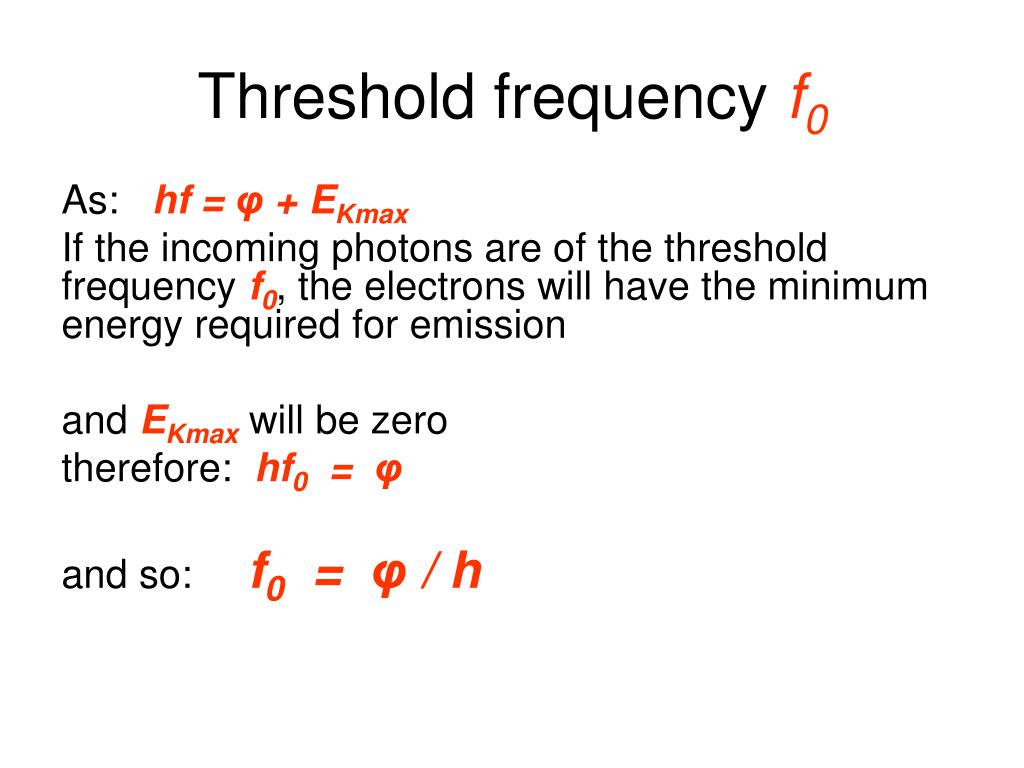
Threshold Energy Unit . Threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from a lower energy. Threshold energy is the minimum amount of energy needed for particles to react. The work function φ, or threshold energy, of a material, is defined as: Consider the electrons in a metal as trapped. Only particles that have at least as much. Threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two reactant molecules. Calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric effect is observed is \(9.87 \times 10^{14}\; For a particular reaction, the threshold energy might be as shown here: What exactly is the definition of threshold energy for a reaction ? For any frequency of light above the threshold frequency, the kinetic energy of an ejected electron will increase linearly with the energy of the incoming photon. Is it the energy that a should have so that so that the heavier of c and d is produced at. The minimum energy required to release a photoelectron from the surface of a metal.
from www.slideserve.com
For a particular reaction, the threshold energy might be as shown here: The work function φ, or threshold energy, of a material, is defined as: Calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric effect is observed is \(9.87 \times 10^{14}\; Consider the electrons in a metal as trapped. The minimum energy required to release a photoelectron from the surface of a metal. Threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from a lower energy. Threshold energy is the minimum amount of energy needed for particles to react. Threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two reactant molecules. What exactly is the definition of threshold energy for a reaction ? For any frequency of light above the threshold frequency, the kinetic energy of an ejected electron will increase linearly with the energy of the incoming photon.
PPT 1.2 Radiation and Quantum Phenomena Quantum
Threshold Energy Unit Threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from a lower energy. Threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two reactant molecules. Threshold energy is the minimum amount of energy needed for particles to react. Consider the electrons in a metal as trapped. For any frequency of light above the threshold frequency, the kinetic energy of an ejected electron will increase linearly with the energy of the incoming photon. What exactly is the definition of threshold energy for a reaction ? Threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from a lower energy. Calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric effect is observed is \(9.87 \times 10^{14}\; For a particular reaction, the threshold energy might be as shown here: Only particles that have at least as much. The minimum energy required to release a photoelectron from the surface of a metal. Is it the energy that a should have so that so that the heavier of c and d is produced at. The work function φ, or threshold energy, of a material, is defined as:
From www.youtube.com
What is activation energy Threshold energy energy barrier rate of Threshold Energy Unit The minimum energy required to release a photoelectron from the surface of a metal. Only particles that have at least as much. Consider the electrons in a metal as trapped. Calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric effect is observed is \(9.87 \times 10^{14}\; For any frequency. Threshold Energy Unit.
From www.vedantu.com
Explain the energy pyramid. Threshold Energy Unit Threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from a lower energy. Is it the energy that a should have so that so that the heavier of c and d is produced at. Threshold energy is the minimum amount of energy needed for particles to react. What. Threshold Energy Unit.
From www.researchgate.net
(colour online) (a) Calculated threshold energy fluence and (b Threshold Energy Unit What exactly is the definition of threshold energy for a reaction ? Threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two reactant molecules. Threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from a lower energy. Only particles that. Threshold Energy Unit.
From www.youtube.com
CHEMISTRY 101 Photoelectric Effect, Threshold Frequency YouTube Threshold Energy Unit Calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric effect is observed is \(9.87 \times 10^{14}\; Threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two reactant molecules. The minimum energy required to release a photoelectron from the surface of a. Threshold Energy Unit.
From www.alamy.com
Graph of Progress of Reaction and Threshold energy Stock Vector Image Threshold Energy Unit Is it the energy that a should have so that so that the heavier of c and d is produced at. For a particular reaction, the threshold energy might be as shown here: The minimum energy required to release a photoelectron from the surface of a metal. Threshold energy is the minimum amount of energy required to initiate a specific. Threshold Energy Unit.
From www.nuclear-power.com
Critical Energy Threshold Energy for Fission Threshold Energy Unit Calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric effect is observed is \(9.87 \times 10^{14}\; Threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two reactant molecules. For any frequency of light above the threshold frequency, the kinetic energy of. Threshold Energy Unit.
From www.researchgate.net
The threshold energy, √ s th , for K + Λ and K + Σ Download Threshold Energy Unit Only particles that have at least as much. Consider the electrons in a metal as trapped. Is it the energy that a should have so that so that the heavier of c and d is produced at. The work function φ, or threshold energy, of a material, is defined as: For a particular reaction, the threshold energy might be as. Threshold Energy Unit.
From www.youtube.com
25) Activation Energy and Threshold Energy Class12 Chemical Threshold Energy Unit Threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from a lower energy. Calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric effect is observed is \(9.87 \times 10^{14}\; Consider the electrons in a metal as trapped.. Threshold Energy Unit.
From howtova.blogspot.com
How To Calculate Threshold Frequency HOWTOVA Threshold Energy Unit The work function φ, or threshold energy, of a material, is defined as: What exactly is the definition of threshold energy for a reaction ? Calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric effect is observed is \(9.87 \times 10^{14}\; Only particles that have at least as much.. Threshold Energy Unit.
From lacey-has-watkins.blogspot.com
Conversion of Units in Physics LaceyhasWatkins Threshold Energy Unit Only particles that have at least as much. Is it the energy that a should have so that so that the heavier of c and d is produced at. Threshold energy is the minimum amount of energy needed for particles to react. Consider the electrons in a metal as trapped. The work function φ, or threshold energy, of a material,. Threshold Energy Unit.
From www.edrawmax.com
What is an Energy Pyramid Diagram EdrawMax Online Threshold Energy Unit Calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric effect is observed is \(9.87 \times 10^{14}\; What exactly is the definition of threshold energy for a reaction ? Only particles that have at least as much. Threshold energy is the minimum amount of energy required to initiate a specific. Threshold Energy Unit.
From www.slideserve.com
PPT RFSS Lecture 9 Nuclear Reactions PowerPoint Presentation, free Threshold Energy Unit For a particular reaction, the threshold energy might be as shown here: The work function φ, or threshold energy, of a material, is defined as: For any frequency of light above the threshold frequency, the kinetic energy of an ejected electron will increase linearly with the energy of the incoming photon. Calculate the threshold energy in kj/mol of electrons in. Threshold Energy Unit.
From byjus.com
What is threshold frequency and threshold energy? Threshold Energy Unit Calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric effect is observed is \(9.87 \times 10^{14}\; Only particles that have at least as much. Is it the energy that a should have so that so that the heavier of c and d is produced at. Consider the electrons in. Threshold Energy Unit.
From www.mrgscience.com
4.2 Energy Flow AMAZING WORLD OF SCIENCE WITH MR. GREEN Threshold Energy Unit Threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two reactant molecules. For any frequency of light above the threshold frequency, the kinetic energy of an ejected electron will increase linearly with the energy of the incoming photon. Consider the electrons in a metal as trapped. The work function φ, or threshold. Threshold Energy Unit.
From www.youtube.com
Explain Q Value and, Threshold energy Nuclear Chemistry Physical Threshold Energy Unit Only particles that have at least as much. Threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two reactant molecules. Is it the energy that a should have so that so that the heavier of c and d is produced at. For a particular reaction, the threshold energy might be as shown. Threshold Energy Unit.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Threshold Energy Unit For any frequency of light above the threshold frequency, the kinetic energy of an ejected electron will increase linearly with the energy of the incoming photon. Threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from a lower energy. Calculate the threshold energy in kj/mol of electrons in. Threshold Energy Unit.
From www.researchgate.net
a The laser threshold energy primary axis and the figureofmerit Threshold Energy Unit For any frequency of light above the threshold frequency, the kinetic energy of an ejected electron will increase linearly with the energy of the incoming photon. Consider the electrons in a metal as trapped. Is it the energy that a should have so that so that the heavier of c and d is produced at. Threshold energy is the minimum. Threshold Energy Unit.
From www.researchgate.net
Threshold (minimum) energy flow for the existence of solitons walking Threshold Energy Unit Threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two reactant molecules. Threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from a lower energy. Is it the energy that a should have so that so that the heavier of. Threshold Energy Unit.
From www.slideserve.com
PPT General Physics (PHY 2140) PowerPoint Presentation, free download Threshold Energy Unit For a particular reaction, the threshold energy might be as shown here: For any frequency of light above the threshold frequency, the kinetic energy of an ejected electron will increase linearly with the energy of the incoming photon. Calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric effect is. Threshold Energy Unit.
From www.slideserve.com
PPT Radioactivity 29.3 PowerPoint Presentation ID2979284 Threshold Energy Unit Is it the energy that a should have so that so that the heavier of c and d is produced at. Consider the electrons in a metal as trapped. The work function φ, or threshold energy, of a material, is defined as: For a particular reaction, the threshold energy might be as shown here: For any frequency of light above. Threshold Energy Unit.
From www.researchgate.net
Variations of remaining energy and threshold energy against prediction Threshold Energy Unit Threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from a lower energy. The work function φ, or threshold energy, of a material, is defined as: Calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric effect is. Threshold Energy Unit.
From www.youtube.com
Concept of Activation & Threshold Energy29Unit 4 chemical Threshold Energy Unit Calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric effect is observed is \(9.87 \times 10^{14}\; The minimum energy required to release a photoelectron from the surface of a metal. Consider the electrons in a metal as trapped. Threshold energy is the minimum amount of energy needed for particles. Threshold Energy Unit.
From www.toppr.com
The energy profile for the reaction CO(g) + NO2(g) CO2(g) + NO(g) is Threshold Energy Unit Is it the energy that a should have so that so that the heavier of c and d is produced at. Threshold energy is the minimum amount of energy needed for particles to react. Threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two reactant molecules. What exactly is the definition of. Threshold Energy Unit.
From www.researchgate.net
The displacement threshold energy E pc d in a perfect Cu crystal at Threshold Energy Unit For a particular reaction, the threshold energy might be as shown here: Calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric effect is observed is \(9.87 \times 10^{14}\; Threshold energy is the minimum amount of energy needed for particles to react. The minimum energy required to release a photoelectron. Threshold Energy Unit.
From byjus.com
What happens when the energy of a reaction if more than threshold Threshold Energy Unit Only particles that have at least as much. The work function φ, or threshold energy, of a material, is defined as: Consider the electrons in a metal as trapped. For a particular reaction, the threshold energy might be as shown here: Threshold energy is the minimum amount of energy needed for particles to react. Calculate the threshold energy in kj/mol. Threshold Energy Unit.
From byjus.com
What happens when the energy of a reaction if more than threshold Threshold Energy Unit For any frequency of light above the threshold frequency, the kinetic energy of an ejected electron will increase linearly with the energy of the incoming photon. The minimum energy required to release a photoelectron from the surface of a metal. Consider the electrons in a metal as trapped. Threshold energy is the minimum amount of energy required to initiate a. Threshold Energy Unit.
From www.youtube.com
Explain the terms Threshold energy 12 CHEMICAL Threshold Energy Unit Calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric effect is observed is \(9.87 \times 10^{14}\; Threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two reactant molecules. Consider the electrons in a metal as trapped. What exactly is the definition. Threshold Energy Unit.
From www.researchgate.net
Solid curve Auger threshold energy E th in units of bandgap Eg in Threshold Energy Unit Calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric effect is observed is \(9.87 \times 10^{14}\; Is it the energy that a should have so that so that the heavier of c and d is produced at. For a particular reaction, the threshold energy might be as shown here:. Threshold Energy Unit.
From www.youtube.com
Threshold Energy Nuclear Fusion and Fission UNIT2 Lect5 Threshold Energy Unit Calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric effect is observed is \(9.87 \times 10^{14}\; What exactly is the definition of threshold energy for a reaction ? Only particles that have at least as much. Consider the electrons in a metal as trapped. The work function φ, or. Threshold Energy Unit.
From www.youtube.com
Revision Threshold Energy UNIT2 PART2 YouTube Threshold Energy Unit What exactly is the definition of threshold energy for a reaction ? Is it the energy that a should have so that so that the heavier of c and d is produced at. The minimum energy required to release a photoelectron from the surface of a metal. The work function φ, or threshold energy, of a material, is defined as:. Threshold Energy Unit.
From socratic.org
What is activation energy? What is threshold energy? What are the Threshold Energy Unit For any frequency of light above the threshold frequency, the kinetic energy of an ejected electron will increase linearly with the energy of the incoming photon. Calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric effect is observed is \(9.87 \times 10^{14}\; The work function φ, or threshold energy,. Threshold Energy Unit.
From byjus.com
26. What is difference between threshold energy and activation energy? Threshold Energy Unit The work function φ, or threshold energy, of a material, is defined as: Threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from a lower energy. For a particular reaction, the threshold energy might be as shown here: Consider the electrons in a metal as trapped. Calculate the. Threshold Energy Unit.
From www.researchgate.net
Threshold energy n of the lowest three subbands as a function of 2. The Threshold Energy Unit Is it the energy that a should have so that so that the heavier of c and d is produced at. Calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric effect is observed is \(9.87 \times 10^{14}\; For a particular reaction, the threshold energy might be as shown here:. Threshold Energy Unit.
From www.youtube.com
Threshold energy & Activation energy by H R Sharma, unit Chemical Threshold Energy Unit The work function φ, or threshold energy, of a material, is defined as: Calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric effect is observed is \(9.87 \times 10^{14}\; Consider the electrons in a metal as trapped. What exactly is the definition of threshold energy for a reaction ?. Threshold Energy Unit.
From www.slideserve.com
PPT 1.2 Radiation and Quantum Phenomena Quantum Threshold Energy Unit The work function φ, or threshold energy, of a material, is defined as: What exactly is the definition of threshold energy for a reaction ? Consider the electrons in a metal as trapped. Calculate the threshold energy in kj/mol of electrons in aluminum, given that the lowest frequency photon for which the photoelectric effect is observed is \(9.87 \times 10^{14}\;. Threshold Energy Unit.