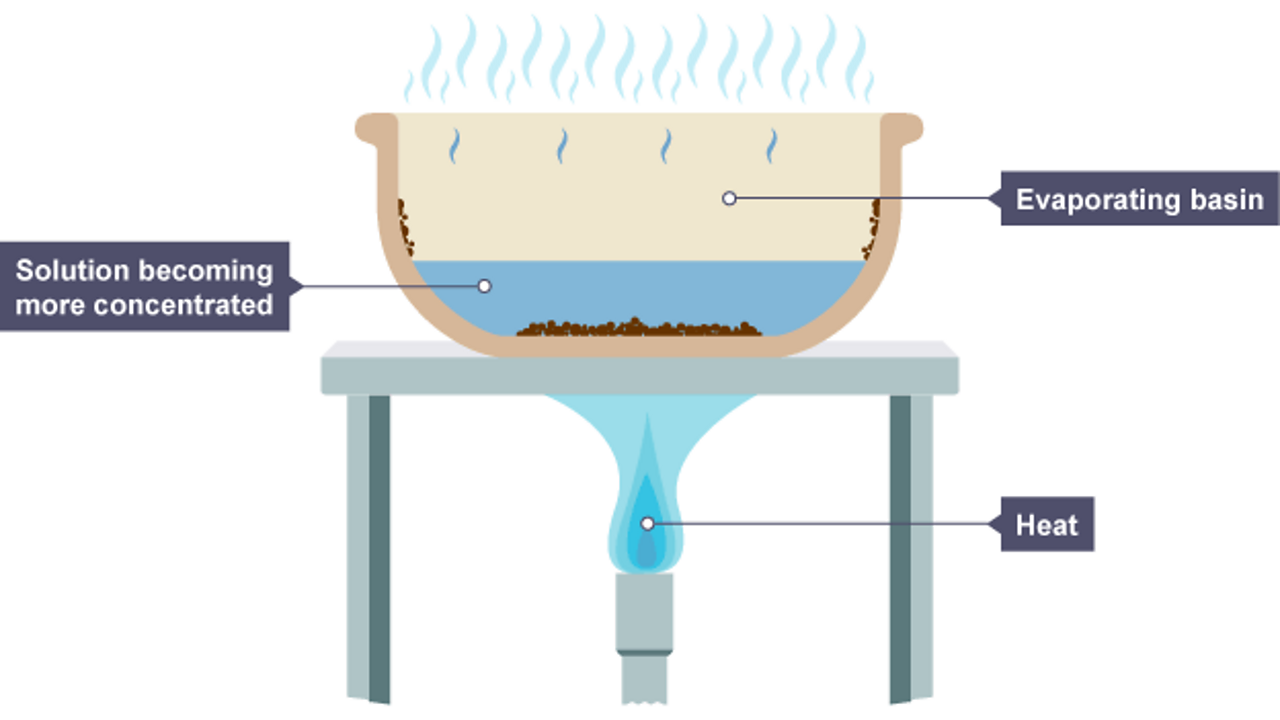
Do Solvents Evaporate . to isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. evaporation rates are used to compare solvents for different reasons and under various conditions. Once the solvent had gone into the gas phase and escaped, we would be left with only the solute. Read on for more information. solvent evaporation is a process where a wall material is dissolved in a continuous phase, and upon evaporation of the. evaporating solvents generally possess one of two benefits when it comes to evaporation rate: They evaporate slowly and have a long exposure. Evaporation from open flasks on a steam bath. The change from liquid phase to gas phase is called evaporation. solvent evaporation is the process of water, or an organic solvent, evaporating and can be carried out via a number of methods. there are three commonly used techniques for solvent evaporation:
from www.bbc.co.uk
evaporation rates are used to compare solvents for different reasons and under various conditions. The change from liquid phase to gas phase is called evaporation. solvent evaporation is a process where a wall material is dissolved in a continuous phase, and upon evaporation of the. Read on for more information. evaporating solvents generally possess one of two benefits when it comes to evaporation rate: They evaporate slowly and have a long exposure. Evaporation from open flasks on a steam bath. to isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. Once the solvent had gone into the gas phase and escaped, we would be left with only the solute. solvent evaporation is the process of water, or an organic solvent, evaporating and can be carried out via a number of methods.
Solutions and Separations BBC Bitesize
Do Solvents Evaporate They evaporate slowly and have a long exposure. evaporating solvents generally possess one of two benefits when it comes to evaporation rate: The change from liquid phase to gas phase is called evaporation. Once the solvent had gone into the gas phase and escaped, we would be left with only the solute. Read on for more information. solvent evaporation is the process of water, or an organic solvent, evaporating and can be carried out via a number of methods. evaporation rates are used to compare solvents for different reasons and under various conditions. there are three commonly used techniques for solvent evaporation: They evaporate slowly and have a long exposure. to isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. solvent evaporation is a process where a wall material is dissolved in a continuous phase, and upon evaporation of the. Evaporation from open flasks on a steam bath.
From www.youtube.com
Evaporate solvents in tubes and vials with ease. The Smart Evaporator Do Solvents Evaporate there are three commonly used techniques for solvent evaporation: Read on for more information. evaporation rates are used to compare solvents for different reasons and under various conditions. Evaporation from open flasks on a steam bath. evaporating solvents generally possess one of two benefits when it comes to evaporation rate: solvent evaporation is a process where. Do Solvents Evaporate.
From www.chegg.com
Solved Prelab 7 Worksheet Why Do Liquids Evaporate at Do Solvents Evaporate evaporation rates are used to compare solvents for different reasons and under various conditions. They evaporate slowly and have a long exposure. Evaporation from open flasks on a steam bath. solvent evaporation is a process where a wall material is dissolved in a continuous phase, and upon evaporation of the. The change from liquid phase to gas phase. Do Solvents Evaporate.
From ecolink.com
Fast Evaporating Solvent Evaporating Degreasers Do Solvents Evaporate Read on for more information. They evaporate slowly and have a long exposure. solvent evaporation is the process of water, or an organic solvent, evaporating and can be carried out via a number of methods. solvent evaporation is a process where a wall material is dissolved in a continuous phase, and upon evaporation of the. Once the solvent. Do Solvents Evaporate.
From www.teachoo.com
Evaporation Meaning and Factors affecting it Teachoo Do Solvents Evaporate there are three commonly used techniques for solvent evaporation: Once the solvent had gone into the gas phase and escaped, we would be left with only the solute. to isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. They evaporate slowly and. Do Solvents Evaporate.
From www.news-medical.net
Guide to solvent evaporation Do Solvents Evaporate solvent evaporation is the process of water, or an organic solvent, evaporating and can be carried out via a number of methods. evaporating solvents generally possess one of two benefits when it comes to evaporation rate: Once the solvent had gone into the gas phase and escaped, we would be left with only the solute. evaporation rates. Do Solvents Evaporate.
From saltworkconsultants.com
Physics of evaporation Do Solvents Evaporate Read on for more information. The change from liquid phase to gas phase is called evaporation. Evaporation from open flasks on a steam bath. to isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. evaporation rates are used to compare solvents for. Do Solvents Evaporate.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Do Solvents Evaporate The change from liquid phase to gas phase is called evaporation. solvent evaporation is the process of water, or an organic solvent, evaporating and can be carried out via a number of methods. They evaporate slowly and have a long exposure. evaporating solvents generally possess one of two benefits when it comes to evaporation rate: evaporation rates. Do Solvents Evaporate.
From www.slideserve.com
PPT Health Hazards in Construction Part 2 PowerPoint Presentation Do Solvents Evaporate Once the solvent had gone into the gas phase and escaped, we would be left with only the solute. there are three commonly used techniques for solvent evaporation: to isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. They evaporate slowly and. Do Solvents Evaporate.
From www.woodsmith.com
A Guide To Finishing Solvents Woodsmith Do Solvents Evaporate solvent evaporation is the process of water, or an organic solvent, evaporating and can be carried out via a number of methods. solvent evaporation is a process where a wall material is dissolved in a continuous phase, and upon evaporation of the. Once the solvent had gone into the gas phase and escaped, we would be left with. Do Solvents Evaporate.
From slideplayer.com
SOLVENTS NOTES This symbol indicates you need to click the mouse Do Solvents Evaporate solvent evaporation is a process where a wall material is dissolved in a continuous phase, and upon evaporation of the. to isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. evaporation rates are used to compare solvents for different reasons and. Do Solvents Evaporate.
From www.researchgate.net
Scheme of the solvent casting process. Download Scientific Diagram Do Solvents Evaporate The change from liquid phase to gas phase is called evaporation. solvent evaporation is the process of water, or an organic solvent, evaporating and can be carried out via a number of methods. They evaporate slowly and have a long exposure. Once the solvent had gone into the gas phase and escaped, we would be left with only the. Do Solvents Evaporate.
From www.expii.com
Good Solvent (Water) — Properties & Examples Expii Do Solvents Evaporate The change from liquid phase to gas phase is called evaporation. solvent evaporation is a process where a wall material is dissolved in a continuous phase, and upon evaporation of the. Read on for more information. solvent evaporation is the process of water, or an organic solvent, evaporating and can be carried out via a number of methods.. Do Solvents Evaporate.
From www.bbc.co.uk
Solutions and Separations BBC Bitesize Do Solvents Evaporate evaporating solvents generally possess one of two benefits when it comes to evaporation rate: Read on for more information. evaporation rates are used to compare solvents for different reasons and under various conditions. solvent evaporation is a process where a wall material is dissolved in a continuous phase, and upon evaporation of the. They evaporate slowly and. Do Solvents Evaporate.
From www.deperu.com
Diagrama infográfico de la solución que muestra el recipiente llenado Do Solvents Evaporate They evaporate slowly and have a long exposure. solvent evaporation is a process where a wall material is dissolved in a continuous phase, and upon evaporation of the. evaporation rates are used to compare solvents for different reasons and under various conditions. evaporating solvents generally possess one of two benefits when it comes to evaporation rate: The. Do Solvents Evaporate.
From masterconceptsinchemistry.com
How does the vapor pressure of solution depend on the concentration of Do Solvents Evaporate there are three commonly used techniques for solvent evaporation: evaporation rates are used to compare solvents for different reasons and under various conditions. to isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. evaporating solvents generally possess one of two. Do Solvents Evaporate.
From www.researchgate.net
Solvent evaporation method of preparation for nanoparticles Download Do Solvents Evaporate The change from liquid phase to gas phase is called evaporation. evaporation rates are used to compare solvents for different reasons and under various conditions. to isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. They evaporate slowly and have a long. Do Solvents Evaporate.
From ecolink.com
Evaporting Solvents Evaporation Rates Industrial Degreasers Do Solvents Evaporate to isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. there are three commonly used techniques for solvent evaporation: Read on for more information. Evaporation from open flasks on a steam bath. evaporation rates are used to compare solvents for different. Do Solvents Evaporate.
From www.coleparmer.fr
Plant Solvent Extraction Method Using Ethanol 3 Steps ColeParmer France Do Solvents Evaporate Read on for more information. solvent evaporation is a process where a wall material is dissolved in a continuous phase, and upon evaporation of the. The change from liquid phase to gas phase is called evaporation. evaporation rates are used to compare solvents for different reasons and under various conditions. They evaporate slowly and have a long exposure.. Do Solvents Evaporate.
From www.vrogue.co
Schematic Diagram Of Solvent Extraction Procedure For vrogue.co Do Solvents Evaporate They evaporate slowly and have a long exposure. evaporating solvents generally possess one of two benefits when it comes to evaporation rate: evaporation rates are used to compare solvents for different reasons and under various conditions. Read on for more information. solvent evaporation is a process where a wall material is dissolved in a continuous phase, and. Do Solvents Evaporate.
From bulkchemicals2go.com
Which Solvents Evaporate the Fastest? BulkChemicals2go Do Solvents Evaporate there are three commonly used techniques for solvent evaporation: Read on for more information. to isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. solvent evaporation is a process where a wall material is dissolved in a continuous phase, and upon. Do Solvents Evaporate.
From www.youtube.com
Chemicals in water Do they really evaporate After 24 Hours? YouTube Do Solvents Evaporate Once the solvent had gone into the gas phase and escaped, we would be left with only the solute. They evaporate slowly and have a long exposure. evaporating solvents generally possess one of two benefits when it comes to evaporation rate: solvent evaporation is the process of water, or an organic solvent, evaporating and can be carried out. Do Solvents Evaporate.
From chemawareness.blogspot.com
Chem Awareness Classification of Solvent`s Types of Solvents Examples, Do Solvents Evaporate evaporating solvents generally possess one of two benefits when it comes to evaporation rate: Once the solvent had gone into the gas phase and escaped, we would be left with only the solute. evaporation rates are used to compare solvents for different reasons and under various conditions. The change from liquid phase to gas phase is called evaporation.. Do Solvents Evaporate.
From www.youtube.com
Evaporating solvents with Nitrogen (2013 version) YouTube Do Solvents Evaporate to isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. Read on for more information. there are three commonly used techniques for solvent evaporation: solvent evaporation is the process of water, or an organic solvent, evaporating and can be carried out. Do Solvents Evaporate.
From www.numerade.com
SOLVED EXPERIMENT 2 EVAPORATION AND INTERMOLECULAR ATTRACTIONS Do Solvents Evaporate Evaporation from open flasks on a steam bath. solvent evaporation is a process where a wall material is dissolved in a continuous phase, and upon evaporation of the. solvent evaporation is the process of water, or an organic solvent, evaporating and can be carried out via a number of methods. evaporating solvents generally possess one of two. Do Solvents Evaporate.
From bulkchemicals2go.com
How Does A Solvent Evaporate? Specialty Chemicals Do Solvents Evaporate They evaporate slowly and have a long exposure. to isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. The change from liquid phase to gas phase is called evaporation. Read on for more information. solvent evaporation is a process where a wall. Do Solvents Evaporate.
From www.aiophotoz.com
What Is Evaporation In The Separation Of Mixtures Images and Photos Do Solvents Evaporate They evaporate slowly and have a long exposure. solvent evaporation is the process of water, or an organic solvent, evaporating and can be carried out via a number of methods. to isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. solvent. Do Solvents Evaporate.
From www.researchgate.net
Color online a Solvent evaporation rate in pure solvents and b Do Solvents Evaporate The change from liquid phase to gas phase is called evaporation. solvent evaporation is a process where a wall material is dissolved in a continuous phase, and upon evaporation of the. there are three commonly used techniques for solvent evaporation: They evaporate slowly and have a long exposure. solvent evaporation is the process of water, or an. Do Solvents Evaporate.
From www.aplustopper.com
How can we Separate a Mixture of a Solid and a Liquid using Evaporation Do Solvents Evaporate evaporation rates are used to compare solvents for different reasons and under various conditions. solvent evaporation is a process where a wall material is dissolved in a continuous phase, and upon evaporation of the. The change from liquid phase to gas phase is called evaporation. there are three commonly used techniques for solvent evaporation: They evaporate slowly. Do Solvents Evaporate.
From dokumen.tips
(PPTX) How Do Liquids Evaporate? DOKUMEN.TIPS Do Solvents Evaporate to isolate the solute, it would be a simple matter of waiting while the solvent molecules all move from the liquid phase to the gas phase. Once the solvent had gone into the gas phase and escaped, we would be left with only the solute. They evaporate slowly and have a long exposure. evaporating solvents generally possess one. Do Solvents Evaporate.
From www.researchgate.net
Solvent evaporation method. (A higher resolution / colour version of Do Solvents Evaporate They evaporate slowly and have a long exposure. The change from liquid phase to gas phase is called evaporation. Read on for more information. evaporating solvents generally possess one of two benefits when it comes to evaporation rate: Once the solvent had gone into the gas phase and escaped, we would be left with only the solute. to. Do Solvents Evaporate.
From www.reddit.com
This is a rotary evaporator. It removes solvents by reducing pressure Do Solvents Evaporate They evaporate slowly and have a long exposure. solvent evaporation is the process of water, or an organic solvent, evaporating and can be carried out via a number of methods. solvent evaporation is a process where a wall material is dissolved in a continuous phase, and upon evaporation of the. to isolate the solute, it would be. Do Solvents Evaporate.
From es.scribd.com
How to Evaporate High Boiling Point Solvents Solvent Vacuum Do Solvents Evaporate there are three commonly used techniques for solvent evaporation: They evaporate slowly and have a long exposure. The change from liquid phase to gas phase is called evaporation. solvent evaporation is the process of water, or an organic solvent, evaporating and can be carried out via a number of methods. evaporation rates are used to compare solvents. Do Solvents Evaporate.
From www.numerade.com
SOLVED EXPERIMENT 2 EVAPORATION AND INTERMOLECULAR ATTRACTIONS Do Solvents Evaporate Evaporation from open flasks on a steam bath. Once the solvent had gone into the gas phase and escaped, we would be left with only the solute. Read on for more information. evaporating solvents generally possess one of two benefits when it comes to evaporation rate: to isolate the solute, it would be a simple matter of waiting. Do Solvents Evaporate.
From www.vrogue.co
What Is Solvent Extraction And Why Is It Important So vrogue.co Do Solvents Evaporate The change from liquid phase to gas phase is called evaporation. solvent evaporation is a process where a wall material is dissolved in a continuous phase, and upon evaporation of the. there are three commonly used techniques for solvent evaporation: Evaporation from open flasks on a steam bath. Once the solvent had gone into the gas phase and. Do Solvents Evaporate.
From hallie-has-cantrell.blogspot.com
Forms When a Solvent Evaporates From a Saturated Solution Halliehas Do Solvents Evaporate Once the solvent had gone into the gas phase and escaped, we would be left with only the solute. solvent evaporation is the process of water, or an organic solvent, evaporating and can be carried out via a number of methods. They evaporate slowly and have a long exposure. evaporation rates are used to compare solvents for different. Do Solvents Evaporate.