How Do You Solve For Standard Enthalpy Of Formation . what is enthalpy (heat) of formation. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the boldfaced values are the coefficients and the other ones are the standard enthalpy of formation for the four substances. This is the enthalpy change for the exothermic reaction:. the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: the standard molar enthalpy of formation δhof is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is formed. Check out a few examples & study its chart. \[ 6c\left (s, graphite \right ) +. Learn its formula, along with a few solved problems. the standard enthalpy of formation of co 2 (g) is −393.5 kj/mol.
from solvedlib.com
the boldfaced values are the coefficients and the other ones are the standard enthalpy of formation for the four substances. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. Check out a few examples & study its chart. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. Learn its formula, along with a few solved problems. This is the enthalpy change for the exothermic reaction:. what is enthalpy (heat) of formation. the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: the standard molar enthalpy of formation δhof is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is formed. the standard enthalpy of formation of co 2 (g) is −393.5 kj/mol.
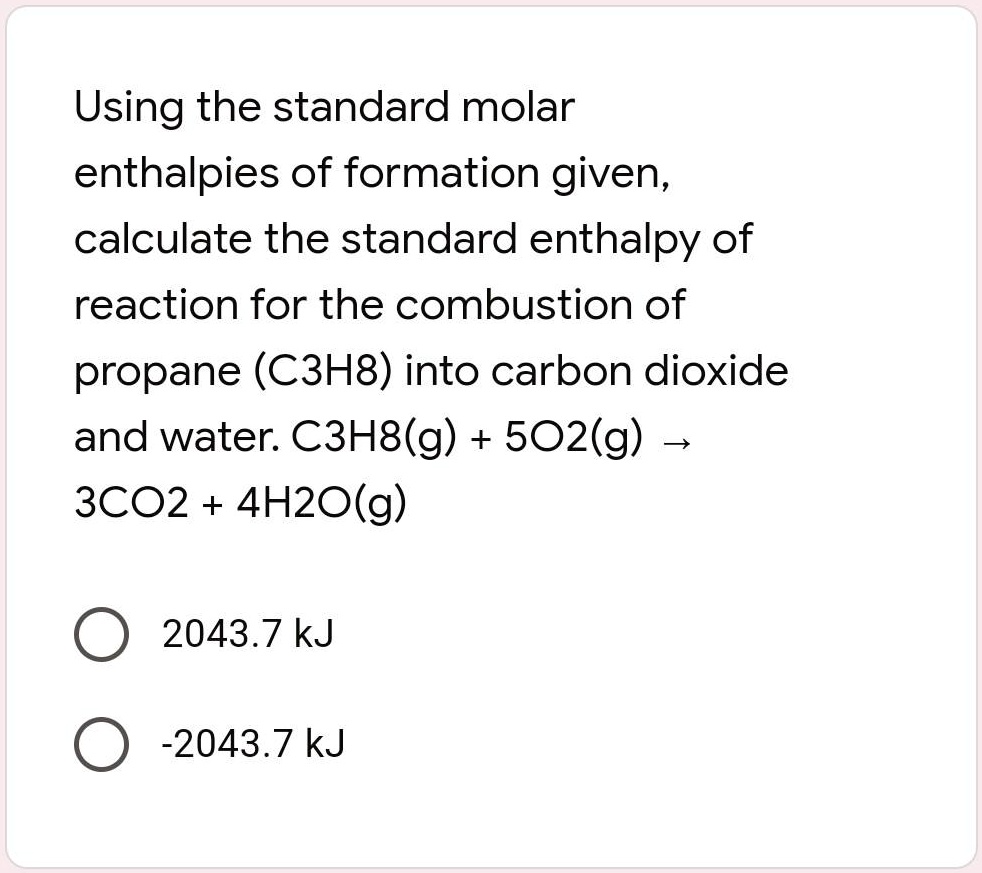
Using the standard molar enthalpies of formation give… SolvedLib
How Do You Solve For Standard Enthalpy Of Formation the standard molar enthalpy of formation δhof is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is formed. the standard molar enthalpy of formation δhof is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is formed. Check out a few examples & study its chart. \[ 6c\left (s, graphite \right ) +. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. what is enthalpy (heat) of formation. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. This is the enthalpy change for the exothermic reaction:. the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: the boldfaced values are the coefficients and the other ones are the standard enthalpy of formation for the four substances. Learn its formula, along with a few solved problems.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy How Do You Solve For Standard Enthalpy Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. what is enthalpy (heat) of formation. \[ 6c\left (s, graphite \right ) +. the standard molar enthalpy of formation δhof is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution,. How Do You Solve For Standard Enthalpy Of Formation.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube How Do You Solve For Standard Enthalpy Of Formation the standard molar enthalpy of formation δhof is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is formed. the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: the standard enthalpy of formation of co 2. How Do You Solve For Standard Enthalpy Of Formation.
From www.toppr.com
Use the given standard enthalpies of formation (in kJ/mol) to determine How Do You Solve For Standard Enthalpy Of Formation the standard molar enthalpy of formation δhof is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is formed. Check out a few examples & study its chart. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the boldfaced. How Do You Solve For Standard Enthalpy Of Formation.
From www.chegg.com
Solved 8) a) Define "standard enthalpy change of formation." How Do You Solve For Standard Enthalpy Of Formation Learn its formula, along with a few solved problems. This is the enthalpy change for the exothermic reaction:. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. what is enthalpy (heat) of formation. the boldfaced values. How Do You Solve For Standard Enthalpy Of Formation.
From www.chegg.com
Solved At 25 degree C, the standard enthalpy of formation of How Do You Solve For Standard Enthalpy Of Formation the boldfaced values are the coefficients and the other ones are the standard enthalpy of formation for the four substances. Check out a few examples & study its chart. This is the enthalpy change for the exothermic reaction:. what is enthalpy (heat) of formation. the standard enthalpy of formation is a measure of the energy released or. How Do You Solve For Standard Enthalpy Of Formation.
From www.chegg.com
Solved Table 1. Standard enthalpies of formation for How Do You Solve For Standard Enthalpy Of Formation the boldfaced values are the coefficients and the other ones are the standard enthalpy of formation for the four substances. This is the enthalpy change for the exothermic reaction:. Check out a few examples & study its chart. the standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. \[ 6c\left (s, graphite \right ) +. Learn. How Do You Solve For Standard Enthalpy Of Formation.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation How Do You Solve For Standard Enthalpy Of Formation the standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: Check out a few examples & study its chart. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation. How Do You Solve For Standard Enthalpy Of Formation.
From www.toppr.com
Calculate the standard enthalpy of formation of CH3 OH from the How Do You Solve For Standard Enthalpy Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. This is the enthalpy change for the exothermic reaction:. Check out a few examples & study its chart. . How Do You Solve For Standard Enthalpy Of Formation.
From www.numerade.com
SOLVED Use the standard enthalpy of formation (ΔHf) values in How Do You Solve For Standard Enthalpy Of Formation the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: what is enthalpy (heat) of formation. the boldfaced values are the coefficients and the other ones are the standard enthalpy of formation for the four substances. the standard enthalpy of formation is a measure of the. How Do You Solve For Standard Enthalpy Of Formation.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube How Do You Solve For Standard Enthalpy Of Formation the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: Check out a few examples & study its chart. the standard molar enthalpy of formation δhof is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is formed.. How Do You Solve For Standard Enthalpy Of Formation.
From studyzoneparker.z19.web.core.windows.net
How To Solve For Enthalpy How Do You Solve For Standard Enthalpy Of Formation the boldfaced values are the coefficients and the other ones are the standard enthalpy of formation for the four substances. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. This is the enthalpy change for the exothermic reaction:. \[ 6c\left (s, graphite \right ) +. the standard molar enthalpy. How Do You Solve For Standard Enthalpy Of Formation.
From www.chegg.com
Solved Calculate the standard enthalpy of formation of How Do You Solve For Standard Enthalpy Of Formation the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: \[ 6c\left (s, graphite \right ) +. the standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. This is the enthalpy change for the exothermic reaction:. the boldfaced values are the coefficients and the other. How Do You Solve For Standard Enthalpy Of Formation.
From www.numerade.com
SOLVED Calculate the standard enthalpy of formation of reaction 2H2(g How Do You Solve For Standard Enthalpy Of Formation \[ 6c\left (s, graphite \right ) +. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. what is enthalpy (heat) of formation. This is the enthalpy change for the exothermic reaction:. . How Do You Solve For Standard Enthalpy Of Formation.
From www.toppr.com
Calculate the standard enthalpy of formation of CH3OH(I) from the How Do You Solve For Standard Enthalpy Of Formation what is enthalpy (heat) of formation. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. Check out a few examples & study its chart. the standard enthalpy of formation of glucose. How Do You Solve For Standard Enthalpy Of Formation.
From www.youtube.com
5.1 Standard enthalpy changes of formation and combustion YouTube How Do You Solve For Standard Enthalpy Of Formation Check out a few examples & study its chart. This is the enthalpy change for the exothermic reaction:. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. what is enthalpy (heat) of formation. the boldfaced values are the coefficients and the other ones are. How Do You Solve For Standard Enthalpy Of Formation.
From www.coursehero.com
[Solved] Use standard enthalpies of formation to calculate the enthalpy How Do You Solve For Standard Enthalpy Of Formation what is enthalpy (heat) of formation. the standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. the standard molar enthalpy of formation δhof is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is formed. the boldfaced values are the coefficients and the other. How Do You Solve For Standard Enthalpy Of Formation.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps How Do You Solve For Standard Enthalpy Of Formation the standard molar enthalpy of formation δhof is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is formed. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. \[ 6c\left (s, graphite \right ) +. the boldfaced values are. How Do You Solve For Standard Enthalpy Of Formation.
From www.toppr.com
Calculate the standard enthalpy of formation of CH3 OH from the How Do You Solve For Standard Enthalpy Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. Learn its formula, along with a few solved problems. This is the enthalpy change for the exothermic reaction:. . How Do You Solve For Standard Enthalpy Of Formation.
From mungfali.com
Standard Enthalpy Change Equation How Do You Solve For Standard Enthalpy Of Formation \[ 6c\left (s, graphite \right ) +. the boldfaced values are the coefficients and the other ones are the standard enthalpy of formation for the four substances. what is enthalpy (heat) of formation. Check out a few examples & study its chart. Learn its formula, along with a few solved problems. the standard molar enthalpy of formation. How Do You Solve For Standard Enthalpy Of Formation.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics How Do You Solve For Standard Enthalpy Of Formation This is the enthalpy change for the exothermic reaction:. \[ 6c\left (s, graphite \right ) +. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. Learn its formula, along with a few solved problems. the boldfaced values are the coefficients and the other ones are the standard enthalpy of formation. How Do You Solve For Standard Enthalpy Of Formation.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all How Do You Solve For Standard Enthalpy Of Formation the standard molar enthalpy of formation δhof is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is formed. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. Check out a few examples & study. How Do You Solve For Standard Enthalpy Of Formation.
From www.chegg.com
Solved Calculate the standard enthalpy of formation for How Do You Solve For Standard Enthalpy Of Formation what is enthalpy (heat) of formation. the standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. This is the enthalpy change for the exothermic reaction:. the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: Learn its formula, along with a few solved problems. . How Do You Solve For Standard Enthalpy Of Formation.
From learningzonegregorin2m.z4.web.core.windows.net
Heat Of Formation Formula How Do You Solve For Standard Enthalpy Of Formation the standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. Check out a few examples & study its chart. Learn its formula, along with a few solved problems. what is enthalpy (heat) of formation. the standard molar enthalpy of formation δhof is the enthalpy change when 1 mole of a pure substance, or a 1. How Do You Solve For Standard Enthalpy Of Formation.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies How Do You Solve For Standard Enthalpy Of Formation \[ 6c\left (s, graphite \right ) +. the standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. Check out a few examples & study its chart. This is the enthalpy change for the exothermic reaction:. the standard molar enthalpy of formation δhof is the enthalpy change when 1 mole of a pure substance, or a 1. How Do You Solve For Standard Enthalpy Of Formation.
From www.slideshare.net
Standard enthalpy of formation How Do You Solve For Standard Enthalpy Of Formation This is the enthalpy change for the exothermic reaction:. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard molar enthalpy of formation δhof is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution,. How Do You Solve For Standard Enthalpy Of Formation.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID How Do You Solve For Standard Enthalpy Of Formation This is the enthalpy change for the exothermic reaction:. the standard molar enthalpy of formation δhof is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is formed. Learn its formula, along with a few solved problems. the standard enthalpy of formation of glucose from the elements at. How Do You Solve For Standard Enthalpy Of Formation.
From www.youtube.com
Standard Enthalpy of Formation and Formation Reactions OpenStax How Do You Solve For Standard Enthalpy Of Formation what is enthalpy (heat) of formation. the boldfaced values are the coefficients and the other ones are the standard enthalpy of formation for the four substances. This is the enthalpy change for the exothermic reaction:. the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: Learn its. How Do You Solve For Standard Enthalpy Of Formation.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free How Do You Solve For Standard Enthalpy Of Formation the standard enthalpy of formation of glucose from the elements at 25°c is the enthalpy change for the following reaction: Learn its formula, along with a few solved problems. what is enthalpy (heat) of formation. the standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. the standard molar enthalpy of formation δhof is the. How Do You Solve For Standard Enthalpy Of Formation.
From www.youtube.com
Standard Enthalpies of Formation Problem (Chemistry) YouTube How Do You Solve For Standard Enthalpy Of Formation the standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. the standard molar enthalpy of formation δhof is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is formed. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation. How Do You Solve For Standard Enthalpy Of Formation.
From dxorvtvqp.blob.core.windows.net
Standard Enthalpy Of Formation Gibbsite at Samuel Speed blog How Do You Solve For Standard Enthalpy Of Formation the boldfaced values are the coefficients and the other ones are the standard enthalpy of formation for the four substances. Learn its formula, along with a few solved problems. the standard molar enthalpy of formation δhof is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is formed.. How Do You Solve For Standard Enthalpy Of Formation.
From solvedlib.com
Using the standard molar enthalpies of formation give… SolvedLib How Do You Solve For Standard Enthalpy Of Formation what is enthalpy (heat) of formation. the standard enthalpy of formation of co 2 (g) is −393.5 kj/mol. Check out a few examples & study its chart. Learn its formula, along with a few solved problems. the boldfaced values are the coefficients and the other ones are the standard enthalpy of formation for the four substances. . How Do You Solve For Standard Enthalpy Of Formation.
From www.youtube.com
6.6 Standard Enthalpy of Formation and Reaction YouTube How Do You Solve For Standard Enthalpy Of Formation what is enthalpy (heat) of formation. This is the enthalpy change for the exothermic reaction:. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. \[ 6c\left (s, graphite \right ) +. the boldfaced values are the coefficients and the other ones are the standard enthalpy of formation for the. How Do You Solve For Standard Enthalpy Of Formation.
From mungfali.com
Enthalpy Change Of Formation Equation How Do You Solve For Standard Enthalpy Of Formation the standard molar enthalpy of formation δhof is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is formed. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation of. How Do You Solve For Standard Enthalpy Of Formation.
From studylib.net
Standard Enthalpy of Formation and Reaction How Do You Solve For Standard Enthalpy Of Formation the boldfaced values are the coefficients and the other ones are the standard enthalpy of formation for the four substances. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of. How Do You Solve For Standard Enthalpy Of Formation.
From mungfali.com
Standard Enthalpy Of Formation Equation How Do You Solve For Standard Enthalpy Of Formation the boldfaced values are the coefficients and the other ones are the standard enthalpy of formation for the four substances. what is enthalpy (heat) of formation. This is the enthalpy change for the exothermic reaction:. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. Learn its formula, along with. How Do You Solve For Standard Enthalpy Of Formation.