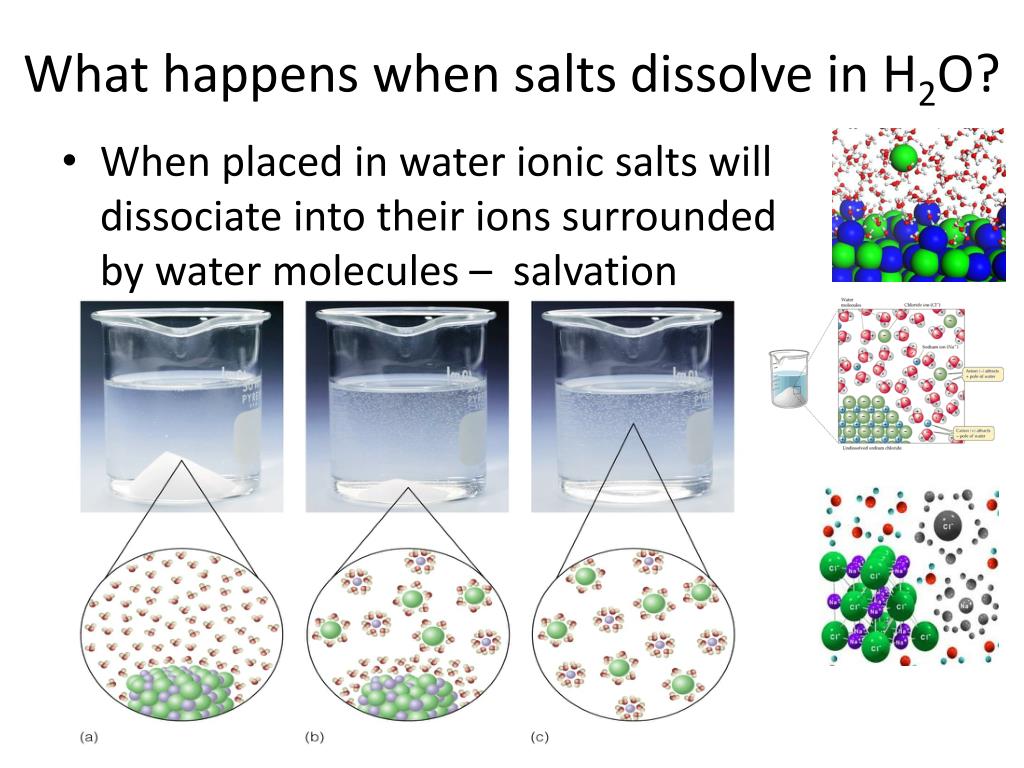
Why Do Salt Crystals Dissolve In Water . The water molecules surround the ions and pull them apart,. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium ions. When salt is added to water, the positive and negative ions separate. You probably have a shaker of. Explain that in this activity, students will investigate how water dissolves salt crystals. If we look at solubility of salts in water, we are told that they disassociate, because the positive na n a ion is attracted to the partially negative oxygen in water and the negative cl c l is. Salt dissolved in water is a rough description of earth's oceans. In chemistry, it results in a solution, as the ionic bond of nacl is pulled apart by the attraction of na to the o of h2o. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water They will also explore how the water from the salt solution evaporates, and how the dissolved salt forms back into crystals again. Bring a pot of water to a boil. Salt, also known as sodium chloride, is one of the most common crystals that readily dissolve in water. Keep stirring (in the boiling water) until the salt.
from www.slideserve.com
You probably have a shaker of. When salt is added to water, the positive and negative ions separate. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water Salt dissolved in water is a rough description of earth's oceans. Salt, also known as sodium chloride, is one of the most common crystals that readily dissolve in water. Explain that in this activity, students will investigate how water dissolves salt crystals. The water molecules surround the ions and pull them apart,. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium ions. In chemistry, it results in a solution, as the ionic bond of nacl is pulled apart by the attraction of na to the o of h2o. If we look at solubility of salts in water, we are told that they disassociate, because the positive na n a ion is attracted to the partially negative oxygen in water and the negative cl c l is.
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free
Why Do Salt Crystals Dissolve In Water If we look at solubility of salts in water, we are told that they disassociate, because the positive na n a ion is attracted to the partially negative oxygen in water and the negative cl c l is. They will also explore how the water from the salt solution evaporates, and how the dissolved salt forms back into crystals again. In chemistry, it results in a solution, as the ionic bond of nacl is pulled apart by the attraction of na to the o of h2o. The water molecules surround the ions and pull them apart,. Keep stirring (in the boiling water) until the salt. Explain that in this activity, students will investigate how water dissolves salt crystals. You probably have a shaker of. If we look at solubility of salts in water, we are told that they disassociate, because the positive na n a ion is attracted to the partially negative oxygen in water and the negative cl c l is. Salt dissolved in water is a rough description of earth's oceans. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water When salt is added to water, the positive and negative ions separate. Bring a pot of water to a boil. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium ions. Salt, also known as sodium chloride, is one of the most common crystals that readily dissolve in water.
From www.pinterest.com
Is Dissolving Salt in Water a Chemical Change or a Physical Change? Why Do Salt Crystals Dissolve In Water Keep stirring (in the boiling water) until the salt. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water You probably have a shaker of. Explain that in this activity, students will investigate how water dissolves salt crystals. Salt, also known as sodium chloride, is one of the most. Why Do Salt Crystals Dissolve In Water.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Why Do Salt Crystals Dissolve In Water In chemistry, it results in a solution, as the ionic bond of nacl is pulled apart by the attraction of na to the o of h2o. You probably have a shaker of. When salt is added to water, the positive and negative ions separate. Explain that in this activity, students will investigate how water dissolves salt crystals. A machine learning. Why Do Salt Crystals Dissolve In Water.
From www.youtube.com
Why Salt Dissolve In Water/உப்பு ஏன் தண்ணீரில் கரைகிறது YouTube Why Do Salt Crystals Dissolve In Water Salt, also known as sodium chloride, is one of the most common crystals that readily dissolve in water. Salt dissolved in water is a rough description of earth's oceans. In chemistry, it results in a solution, as the ionic bond of nacl is pulled apart by the attraction of na to the o of h2o. The water molecules surround the. Why Do Salt Crystals Dissolve In Water.
From www.vectorstock.com
How does sodium chloride nacl dissolve in water Vector Image Why Do Salt Crystals Dissolve In Water Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium ions. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water Salt, also known as sodium chloride, is one of the most. Why Do Salt Crystals Dissolve In Water.
From www.coursehero.com
[Solved] 12. Using diagrams, demonstrate what happens when sodium Why Do Salt Crystals Dissolve In Water A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water Salt, also known as sodium chloride, is one of the most common crystals that readily dissolve in water. Bring a pot of water to a boil. Keep stirring (in the boiling water) until the salt. The water molecules surround. Why Do Salt Crystals Dissolve In Water.
From www.youtube.com
salt crystals dissolving in water under a microscope 2 YouTube Why Do Salt Crystals Dissolve In Water They will also explore how the water from the salt solution evaporates, and how the dissolved salt forms back into crystals again. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium ions. In chemistry, it results in a solution, as the ionic. Why Do Salt Crystals Dissolve In Water.
From www.youtube.com
Salt dissolves in water Is this a chemical or physical change? YouTube Why Do Salt Crystals Dissolve In Water In chemistry, it results in a solution, as the ionic bond of nacl is pulled apart by the attraction of na to the o of h2o. Salt dissolved in water is a rough description of earth's oceans. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water You probably. Why Do Salt Crystals Dissolve In Water.
From visionlearning.com
Solutions, Solubility, and Colligative Properties Chemistry Why Do Salt Crystals Dissolve In Water The water molecules surround the ions and pull them apart,. If we look at solubility of salts in water, we are told that they disassociate, because the positive na n a ion is attracted to the partially negative oxygen in water and the negative cl c l is. They will also explore how the water from the salt solution evaporates,. Why Do Salt Crystals Dissolve In Water.
From loeiulzbj.blob.core.windows.net
Why The Salt Dissolves In Water at Beverly Leach blog Why Do Salt Crystals Dissolve In Water Explain that in this activity, students will investigate how water dissolves salt crystals. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium ions. In chemistry, it results in a solution, as the ionic bond of nacl is pulled apart by the attraction. Why Do Salt Crystals Dissolve In Water.
From www.aquriousmind.com
Why does salt dissolve in water? AQuriousMind Why Do Salt Crystals Dissolve In Water Salt dissolved in water is a rough description of earth's oceans. When salt is added to water, the positive and negative ions separate. Salt, also known as sodium chloride, is one of the most common crystals that readily dissolve in water. Keep stirring (in the boiling water) until the salt. They will also explore how the water from the salt. Why Do Salt Crystals Dissolve In Water.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID762021 Why Do Salt Crystals Dissolve In Water Explain that in this activity, students will investigate how water dissolves salt crystals. The water molecules surround the ions and pull them apart,. They will also explore how the water from the salt solution evaporates, and how the dissolved salt forms back into crystals again. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then. Why Do Salt Crystals Dissolve In Water.
From www.expii.com
Good Solvent (Water) — Properties & Examples Expii Why Do Salt Crystals Dissolve In Water Keep stirring (in the boiling water) until the salt. Salt dissolved in water is a rough description of earth's oceans. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium ions. They will also explore how the water from the salt solution evaporates,. Why Do Salt Crystals Dissolve In Water.
From philschatz.com
Compounds Essential to Human Functioning · Anatomy and Physiology Why Do Salt Crystals Dissolve In Water They will also explore how the water from the salt solution evaporates, and how the dissolved salt forms back into crystals again. Salt dissolved in water is a rough description of earth's oceans. Salt, also known as sodium chloride, is one of the most common crystals that readily dissolve in water. Water can dissolve salt because the positive part of. Why Do Salt Crystals Dissolve In Water.
From mungfali.com
Dissolving Salt In Water Diagram Why Do Salt Crystals Dissolve In Water Keep stirring (in the boiling water) until the salt. If we look at solubility of salts in water, we are told that they disassociate, because the positive na n a ion is attracted to the partially negative oxygen in water and the negative cl c l is. In chemistry, it results in a solution, as the ionic bond of nacl. Why Do Salt Crystals Dissolve In Water.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Why Do Salt Crystals Dissolve In Water They will also explore how the water from the salt solution evaporates, and how the dissolved salt forms back into crystals again. Salt dissolved in water is a rough description of earth's oceans. Keep stirring (in the boiling water) until the salt. The water molecules surround the ions and pull them apart,. In chemistry, it results in a solution, as. Why Do Salt Crystals Dissolve In Water.
From fphoto.photoshelter.com
science chemistry experiment solubility Fundamental Photographs The Why Do Salt Crystals Dissolve In Water Salt, also known as sodium chloride, is one of the most common crystals that readily dissolve in water. The water molecules surround the ions and pull them apart,. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water Keep stirring (in the boiling water) until the salt. They will. Why Do Salt Crystals Dissolve In Water.
From www.youtube.com
How does salt dissolve in water? YouTube Why Do Salt Crystals Dissolve In Water In chemistry, it results in a solution, as the ionic bond of nacl is pulled apart by the attraction of na to the o of h2o. When salt is added to water, the positive and negative ions separate. They will also explore how the water from the salt solution evaporates, and how the dissolved salt forms back into crystals again.. Why Do Salt Crystals Dissolve In Water.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Why Do Salt Crystals Dissolve In Water Salt dissolved in water is a rough description of earth's oceans. Salt, also known as sodium chloride, is one of the most common crystals that readily dissolve in water. The water molecules surround the ions and pull them apart,. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of. Why Do Salt Crystals Dissolve In Water.
From eduvik.in
Matter in Our Surroundings Class 9 Notes Science Chapter 1 Eduvik Why Do Salt Crystals Dissolve In Water You probably have a shaker of. Salt, also known as sodium chloride, is one of the most common crystals that readily dissolve in water. Explain that in this activity, students will investigate how water dissolves salt crystals. Keep stirring (in the boiling water) until the salt. The water molecules surround the ions and pull them apart,. If we look at. Why Do Salt Crystals Dissolve In Water.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Why Do Salt Crystals Dissolve In Water When salt is added to water, the positive and negative ions separate. Explain that in this activity, students will investigate how water dissolves salt crystals. The water molecules surround the ions and pull them apart,. Salt dissolved in water is a rough description of earth's oceans. Keep stirring (in the boiling water) until the salt. Salt, also known as sodium. Why Do Salt Crystals Dissolve In Water.
From fphoto.photoshelter.com
science chemistry experiment solubility Fundamental Photographs The Why Do Salt Crystals Dissolve In Water You probably have a shaker of. Salt dissolved in water is a rough description of earth's oceans. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium ions. Keep stirring (in the boiling water) until the salt. Explain that in this activity, students. Why Do Salt Crystals Dissolve In Water.
From users.highland.edu
Aqueous Solutions Why Do Salt Crystals Dissolve In Water Explain that in this activity, students will investigate how water dissolves salt crystals. The water molecules surround the ions and pull them apart,. Salt, also known as sodium chloride, is one of the most common crystals that readily dissolve in water. Keep stirring (in the boiling water) until the salt. They will also explore how the water from the salt. Why Do Salt Crystals Dissolve In Water.
From www.wyzant.com
Why does salt crystals dissolve in the water? Wyzant Ask An Expert Why Do Salt Crystals Dissolve In Water Salt dissolved in water is a rough description of earth's oceans. Salt, also known as sodium chloride, is one of the most common crystals that readily dissolve in water. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium ions. Explain that in. Why Do Salt Crystals Dissolve In Water.
From butjustwhy.com
Why does salt dissolve in water? But Just Why!? Why Do Salt Crystals Dissolve In Water Salt, also known as sodium chloride, is one of the most common crystals that readily dissolve in water. Keep stirring (in the boiling water) until the salt. Explain that in this activity, students will investigate how water dissolves salt crystals. You probably have a shaker of. Bring a pot of water to a boil. If we look at solubility of. Why Do Salt Crystals Dissolve In Water.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free Why Do Salt Crystals Dissolve In Water Explain that in this activity, students will investigate how water dissolves salt crystals. When salt is added to water, the positive and negative ions separate. In chemistry, it results in a solution, as the ionic bond of nacl is pulled apart by the attraction of na to the o of h2o. Keep stirring (in the boiling water) until the salt.. Why Do Salt Crystals Dissolve In Water.
From fphoto.photoshelter.com
science chemistry experiment solubility Fundamental Photographs The Why Do Salt Crystals Dissolve In Water They will also explore how the water from the salt solution evaporates, and how the dissolved salt forms back into crystals again. Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium ions. A machine learning model has revealed how crystals of sodium. Why Do Salt Crystals Dissolve In Water.
From www.slideserve.com
PPT Chemical Reactions Chapter 7 PowerPoint Presentation, free Why Do Salt Crystals Dissolve In Water If we look at solubility of salts in water, we are told that they disassociate, because the positive na n a ion is attracted to the partially negative oxygen in water and the negative cl c l is. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water In. Why Do Salt Crystals Dissolve In Water.
From www.youtube.com
How Salt Dissolves in Water? YouTube Why Do Salt Crystals Dissolve In Water Keep stirring (in the boiling water) until the salt. Explain that in this activity, students will investigate how water dissolves salt crystals. In chemistry, it results in a solution, as the ionic bond of nacl is pulled apart by the attraction of na to the o of h2o. When salt is added to water, the positive and negative ions separate.. Why Do Salt Crystals Dissolve In Water.
From www.slideserve.com
PPT SPONCH PowerPoint Presentation, free download ID556676 Why Do Salt Crystals Dissolve In Water When salt is added to water, the positive and negative ions separate. Keep stirring (in the boiling water) until the salt. In chemistry, it results in a solution, as the ionic bond of nacl is pulled apart by the attraction of na to the o of h2o. You probably have a shaker of. They will also explore how the water. Why Do Salt Crystals Dissolve In Water.
From studylibstearine.z21.web.core.windows.net
How Does Water Dissolve Salt Why Do Salt Crystals Dissolve In Water If we look at solubility of salts in water, we are told that they disassociate, because the positive na n a ion is attracted to the partially negative oxygen in water and the negative cl c l is. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water Keep. Why Do Salt Crystals Dissolve In Water.
From masterconceptsinchemistry.com
How does sodium chloride (NaCl) dissolve in water? Why Do Salt Crystals Dissolve In Water Keep stirring (in the boiling water) until the salt. They will also explore how the water from the salt solution evaporates, and how the dissolved salt forms back into crystals again. In chemistry, it results in a solution, as the ionic bond of nacl is pulled apart by the attraction of na to the o of h2o. The water molecules. Why Do Salt Crystals Dissolve In Water.
From mungfali.com
Dissolving Salt In Water Diagram Why Do Salt Crystals Dissolve In Water Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium ions. When salt is added to water, the positive and negative ions separate. Salt, also known as sodium chloride, is one of the most common crystals that readily dissolve in water. Explain that. Why Do Salt Crystals Dissolve In Water.
From www.findlight.net
XRay Diffraction Getting to Know Crystal Structures (Part Ⅰ) Why Do Salt Crystals Dissolve In Water A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly crumble to dissolve in water They will also explore how the water from the salt solution evaporates, and how the dissolved salt forms back into crystals again. Explain that in this activity, students will investigate how water dissolves salt crystals. You probably have a. Why Do Salt Crystals Dissolve In Water.
From www.slideserve.com
PPT Chapter 13 Solutions PowerPoint Presentation, free download ID Why Do Salt Crystals Dissolve In Water When salt is added to water, the positive and negative ions separate. They will also explore how the water from the salt solution evaporates, and how the dissolved salt forms back into crystals again. Bring a pot of water to a boil. Keep stirring (in the boiling water) until the salt. Salt, also known as sodium chloride, is one of. Why Do Salt Crystals Dissolve In Water.
From www.slideserve.com
PPT melting & boiling points PowerPoint Presentation, free download Why Do Salt Crystals Dissolve In Water If we look at solubility of salts in water, we are told that they disassociate, because the positive na n a ion is attracted to the partially negative oxygen in water and the negative cl c l is. You probably have a shaker of. A machine learning model has revealed how crystals of sodium chloride slowly weaken and then rapidly. Why Do Salt Crystals Dissolve In Water.