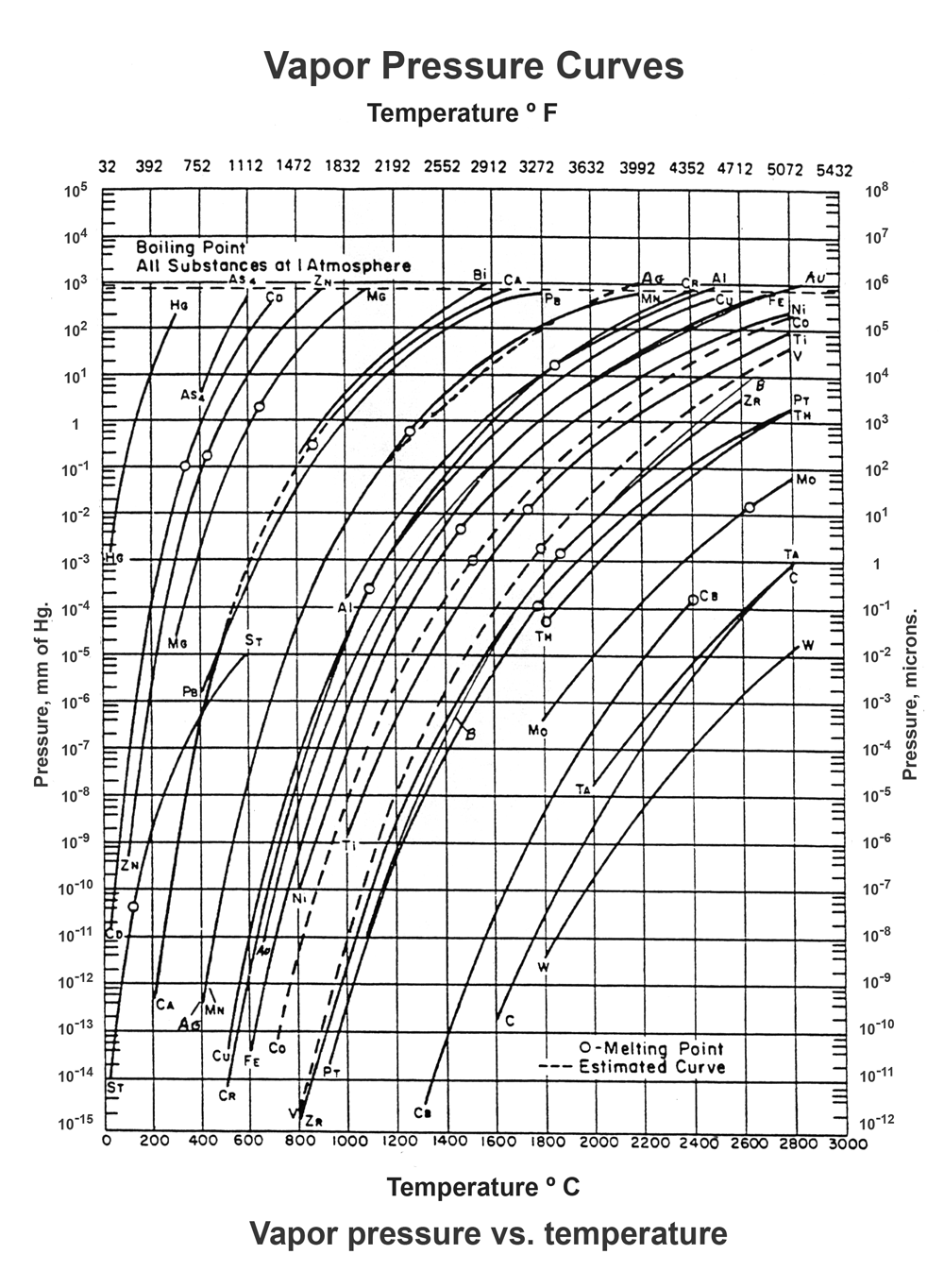
Effect Of Pressure On Evaporation . according to the classical scatterplot, the relationship between pressure and evaporation was not strong. evaporation of a liquid body into a gas phase (air, in the present discussion) is spontaneous if the gas. explain the relationship between vapor pressure of water and the capacity of air to hold water vapor. Calculate humidity and dew point. this process, called vaporization or evaporation, generates a vapor pressure above the liquid. Explain the relationship between relative humidity and partial pressure of water vapor in the air. Calculate vapor density using vapor pressure. the main factors are air and water temperature, relative humidity, wind velocity, surface area, atmospheric. the evaporation rate of a substance is positively associated with the difference between vapor pressure and. Molecules in the gas phase can collide with the liquid surface and reenter the liquid via condensation.
from vacaero.com
Calculate humidity and dew point. the evaporation rate of a substance is positively associated with the difference between vapor pressure and. this process, called vaporization or evaporation, generates a vapor pressure above the liquid. the main factors are air and water temperature, relative humidity, wind velocity, surface area, atmospheric. according to the classical scatterplot, the relationship between pressure and evaporation was not strong. Calculate vapor density using vapor pressure. evaporation of a liquid body into a gas phase (air, in the present discussion) is spontaneous if the gas. explain the relationship between vapor pressure of water and the capacity of air to hold water vapor. Molecules in the gas phase can collide with the liquid surface and reenter the liquid via condensation. Explain the relationship between relative humidity and partial pressure of water vapor in the air.
Evaporation
Effect Of Pressure On Evaporation evaporation of a liquid body into a gas phase (air, in the present discussion) is spontaneous if the gas. the main factors are air and water temperature, relative humidity, wind velocity, surface area, atmospheric. according to the classical scatterplot, the relationship between pressure and evaporation was not strong. evaporation of a liquid body into a gas phase (air, in the present discussion) is spontaneous if the gas. this process, called vaporization or evaporation, generates a vapor pressure above the liquid. Molecules in the gas phase can collide with the liquid surface and reenter the liquid via condensation. explain the relationship between vapor pressure of water and the capacity of air to hold water vapor. Explain the relationship between relative humidity and partial pressure of water vapor in the air. the evaporation rate of a substance is positively associated with the difference between vapor pressure and. Calculate humidity and dew point. Calculate vapor density using vapor pressure.
From www.researchgate.net
Evolution of water vapor pressure Pvapor along with interfacial Effect Of Pressure On Evaporation Explain the relationship between relative humidity and partial pressure of water vapor in the air. Calculate humidity and dew point. the main factors are air and water temperature, relative humidity, wind velocity, surface area, atmospheric. Calculate vapor density using vapor pressure. Molecules in the gas phase can collide with the liquid surface and reenter the liquid via condensation. . Effect Of Pressure On Evaporation.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 Effect Of Pressure On Evaporation according to the classical scatterplot, the relationship between pressure and evaporation was not strong. Molecules in the gas phase can collide with the liquid surface and reenter the liquid via condensation. Calculate humidity and dew point. this process, called vaporization or evaporation, generates a vapor pressure above the liquid. Explain the relationship between relative humidity and partial pressure. Effect Of Pressure On Evaporation.
From www.semanticscholar.org
Figure 1 from The Effects of the Atmospheric Pressure on Evaporation Effect Of Pressure On Evaporation Calculate humidity and dew point. this process, called vaporization or evaporation, generates a vapor pressure above the liquid. Molecules in the gas phase can collide with the liquid surface and reenter the liquid via condensation. the main factors are air and water temperature, relative humidity, wind velocity, surface area, atmospheric. explain the relationship between vapor pressure of. Effect Of Pressure On Evaporation.
From www.researchgate.net
Effects of evaporation coefficient τ lv on (a) pressure, (b) mass flow Effect Of Pressure On Evaporation the main factors are air and water temperature, relative humidity, wind velocity, surface area, atmospheric. evaporation of a liquid body into a gas phase (air, in the present discussion) is spontaneous if the gas. Explain the relationship between relative humidity and partial pressure of water vapor in the air. Calculate humidity and dew point. explain the relationship. Effect Of Pressure On Evaporation.
From www.youtube.com
Effect of pressure in the state of change of matter ! Evaporation and Effect Of Pressure On Evaporation Calculate humidity and dew point. this process, called vaporization or evaporation, generates a vapor pressure above the liquid. Molecules in the gas phase can collide with the liquid surface and reenter the liquid via condensation. the evaporation rate of a substance is positively associated with the difference between vapor pressure and. explain the relationship between vapor pressure. Effect Of Pressure On Evaporation.
From www.teachoo.com
Evaporation Meaning and Factors affecting it Teachoo Effect Of Pressure On Evaporation the main factors are air and water temperature, relative humidity, wind velocity, surface area, atmospheric. according to the classical scatterplot, the relationship between pressure and evaporation was not strong. the evaporation rate of a substance is positively associated with the difference between vapor pressure and. explain the relationship between vapor pressure of water and the capacity. Effect Of Pressure On Evaporation.
From www.slideserve.com
PPT Part AUnit 2 PowerPoint Presentation, free download ID2484640 Effect Of Pressure On Evaporation Calculate humidity and dew point. the main factors are air and water temperature, relative humidity, wind velocity, surface area, atmospheric. the evaporation rate of a substance is positively associated with the difference between vapor pressure and. this process, called vaporization or evaporation, generates a vapor pressure above the liquid. explain the relationship between vapor pressure of. Effect Of Pressure On Evaporation.
From www.slideshare.net
Evaporation Effect Of Pressure On Evaporation this process, called vaporization or evaporation, generates a vapor pressure above the liquid. Calculate humidity and dew point. Calculate vapor density using vapor pressure. the main factors are air and water temperature, relative humidity, wind velocity, surface area, atmospheric. Molecules in the gas phase can collide with the liquid surface and reenter the liquid via condensation. according. Effect Of Pressure On Evaporation.
From www.researchgate.net
presents the effect of evaporation pressure (p3) on the compression Effect Of Pressure On Evaporation Molecules in the gas phase can collide with the liquid surface and reenter the liquid via condensation. Calculate vapor density using vapor pressure. Explain the relationship between relative humidity and partial pressure of water vapor in the air. according to the classical scatterplot, the relationship between pressure and evaporation was not strong. explain the relationship between vapor pressure. Effect Of Pressure On Evaporation.
From www.researchgate.net
Evaporation rate against the vapour pressure difference for various Effect Of Pressure On Evaporation according to the classical scatterplot, the relationship between pressure and evaporation was not strong. this process, called vaporization or evaporation, generates a vapor pressure above the liquid. Calculate humidity and dew point. the main factors are air and water temperature, relative humidity, wind velocity, surface area, atmospheric. evaporation of a liquid body into a gas phase. Effect Of Pressure On Evaporation.
From www.youtube.com
Effect of pressure Change of state from gas to liquid CBSE Class 9 Effect Of Pressure On Evaporation Molecules in the gas phase can collide with the liquid surface and reenter the liquid via condensation. Calculate humidity and dew point. Explain the relationship between relative humidity and partial pressure of water vapor in the air. explain the relationship between vapor pressure of water and the capacity of air to hold water vapor. the evaporation rate of. Effect Of Pressure On Evaporation.
From www.youtube.com
Evaporation Calculations SingleEffect Evaporation Calculations Effect Of Pressure On Evaporation explain the relationship between vapor pressure of water and the capacity of air to hold water vapor. the main factors are air and water temperature, relative humidity, wind velocity, surface area, atmospheric. Molecules in the gas phase can collide with the liquid surface and reenter the liquid via condensation. this process, called vaporization or evaporation, generates a. Effect Of Pressure On Evaporation.
From www.researchgate.net
Effect of the evaporation pressure of different organic working fluids Effect Of Pressure On Evaporation according to the classical scatterplot, the relationship between pressure and evaporation was not strong. Molecules in the gas phase can collide with the liquid surface and reenter the liquid via condensation. evaporation of a liquid body into a gas phase (air, in the present discussion) is spontaneous if the gas. Explain the relationship between relative humidity and partial. Effect Of Pressure On Evaporation.
From www.researchgate.net
Effect of evaporation pressure on the exergoeconomic indicators ab Effect Of Pressure On Evaporation according to the classical scatterplot, the relationship between pressure and evaporation was not strong. Molecules in the gas phase can collide with the liquid surface and reenter the liquid via condensation. Calculate humidity and dew point. this process, called vaporization or evaporation, generates a vapor pressure above the liquid. the main factors are air and water temperature,. Effect Of Pressure On Evaporation.
From www.researchgate.net
Effect of evaporation temperature on performances of dual pressure Effect Of Pressure On Evaporation Molecules in the gas phase can collide with the liquid surface and reenter the liquid via condensation. this process, called vaporization or evaporation, generates a vapor pressure above the liquid. according to the classical scatterplot, the relationship between pressure and evaporation was not strong. explain the relationship between vapor pressure of water and the capacity of air. Effect Of Pressure On Evaporation.
From www.researchgate.net
For two different evaporation temperatures the dependence of the Effect Of Pressure On Evaporation Explain the relationship between relative humidity and partial pressure of water vapor in the air. according to the classical scatterplot, the relationship between pressure and evaporation was not strong. Calculate humidity and dew point. this process, called vaporization or evaporation, generates a vapor pressure above the liquid. the evaporation rate of a substance is positively associated with. Effect Of Pressure On Evaporation.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Effect Of Pressure On Evaporation according to the classical scatterplot, the relationship between pressure and evaporation was not strong. Explain the relationship between relative humidity and partial pressure of water vapor in the air. this process, called vaporization or evaporation, generates a vapor pressure above the liquid. the main factors are air and water temperature, relative humidity, wind velocity, surface area, atmospheric.. Effect Of Pressure On Evaporation.
From www.slideserve.com
PPT Vaporization PowerPoint Presentation, free download ID3207099 Effect Of Pressure On Evaporation this process, called vaporization or evaporation, generates a vapor pressure above the liquid. Molecules in the gas phase can collide with the liquid surface and reenter the liquid via condensation. Explain the relationship between relative humidity and partial pressure of water vapor in the air. the evaporation rate of a substance is positively associated with the difference between. Effect Of Pressure On Evaporation.
From www.youtube.com
Class 9th, Science, Effect of Pressure & evaporation, Chapter 1st Effect Of Pressure On Evaporation Calculate vapor density using vapor pressure. explain the relationship between vapor pressure of water and the capacity of air to hold water vapor. Calculate humidity and dew point. Explain the relationship between relative humidity and partial pressure of water vapor in the air. this process, called vaporization or evaporation, generates a vapor pressure above the liquid. according. Effect Of Pressure On Evaporation.
From www.teachoo.com
Evaporation Meaning and Factors affecting it Teachoo Effect Of Pressure On Evaporation evaporation of a liquid body into a gas phase (air, in the present discussion) is spontaneous if the gas. Calculate humidity and dew point. Molecules in the gas phase can collide with the liquid surface and reenter the liquid via condensation. Explain the relationship between relative humidity and partial pressure of water vapor in the air. according to. Effect Of Pressure On Evaporation.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube Effect Of Pressure On Evaporation Calculate vapor density using vapor pressure. according to the classical scatterplot, the relationship between pressure and evaporation was not strong. this process, called vaporization or evaporation, generates a vapor pressure above the liquid. evaporation of a liquid body into a gas phase (air, in the present discussion) is spontaneous if the gas. explain the relationship between. Effect Of Pressure On Evaporation.
From www.researchgate.net
presents the effect of evaporation pressure (p3) on the compression Effect Of Pressure On Evaporation the evaporation rate of a substance is positively associated with the difference between vapor pressure and. evaporation of a liquid body into a gas phase (air, in the present discussion) is spontaneous if the gas. Calculate humidity and dew point. the main factors are air and water temperature, relative humidity, wind velocity, surface area, atmospheric. this. Effect Of Pressure On Evaporation.
From pharmacyscope.com
Vapour pressure Pharmacy Scope Effect Of Pressure On Evaporation Calculate vapor density using vapor pressure. this process, called vaporization or evaporation, generates a vapor pressure above the liquid. evaporation of a liquid body into a gas phase (air, in the present discussion) is spontaneous if the gas. Molecules in the gas phase can collide with the liquid surface and reenter the liquid via condensation. according to. Effect Of Pressure On Evaporation.
From www.youtube.com
Evaporation , condensation pressure , equilibirium vapour pressure Effect Of Pressure On Evaporation explain the relationship between vapor pressure of water and the capacity of air to hold water vapor. Molecules in the gas phase can collide with the liquid surface and reenter the liquid via condensation. evaporation of a liquid body into a gas phase (air, in the present discussion) is spontaneous if the gas. according to the classical. Effect Of Pressure On Evaporation.
From www.teachoo.com
Changing Pressure to Change State of Matter Chemistry Teachoo Effect Of Pressure On Evaporation Calculate humidity and dew point. the evaporation rate of a substance is positively associated with the difference between vapor pressure and. Explain the relationship between relative humidity and partial pressure of water vapor in the air. the main factors are air and water temperature, relative humidity, wind velocity, surface area, atmospheric. Molecules in the gas phase can collide. Effect Of Pressure On Evaporation.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Effect Of Pressure On Evaporation Calculate vapor density using vapor pressure. explain the relationship between vapor pressure of water and the capacity of air to hold water vapor. Calculate humidity and dew point. the evaporation rate of a substance is positively associated with the difference between vapor pressure and. according to the classical scatterplot, the relationship between pressure and evaporation was not. Effect Of Pressure On Evaporation.
From vacaero.com
Evaporation Effect Of Pressure On Evaporation Molecules in the gas phase can collide with the liquid surface and reenter the liquid via condensation. evaporation of a liquid body into a gas phase (air, in the present discussion) is spontaneous if the gas. explain the relationship between vapor pressure of water and the capacity of air to hold water vapor. Explain the relationship between relative. Effect Of Pressure On Evaporation.
From giokysztp.blob.core.windows.net
Does Pressure Affect Evaporation at Lily Salcedo blog Effect Of Pressure On Evaporation Molecules in the gas phase can collide with the liquid surface and reenter the liquid via condensation. Explain the relationship between relative humidity and partial pressure of water vapor in the air. explain the relationship between vapor pressure of water and the capacity of air to hold water vapor. Calculate humidity and dew point. Calculate vapor density using vapor. Effect Of Pressure On Evaporation.
From www.researchgate.net
Comparison of evaporation rate versus surface pressure head (a Effect Of Pressure On Evaporation Explain the relationship between relative humidity and partial pressure of water vapor in the air. the evaporation rate of a substance is positively associated with the difference between vapor pressure and. Molecules in the gas phase can collide with the liquid surface and reenter the liquid via condensation. Calculate vapor density using vapor pressure. explain the relationship between. Effect Of Pressure On Evaporation.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 Effect Of Pressure On Evaporation Calculate vapor density using vapor pressure. Explain the relationship between relative humidity and partial pressure of water vapor in the air. the evaporation rate of a substance is positively associated with the difference between vapor pressure and. the main factors are air and water temperature, relative humidity, wind velocity, surface area, atmospheric. Molecules in the gas phase can. Effect Of Pressure On Evaporation.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint Effect Of Pressure On Evaporation the evaporation rate of a substance is positively associated with the difference between vapor pressure and. according to the classical scatterplot, the relationship between pressure and evaporation was not strong. Calculate vapor density using vapor pressure. evaporation of a liquid body into a gas phase (air, in the present discussion) is spontaneous if the gas. explain. Effect Of Pressure On Evaporation.
From www.semanticscholar.org
Figure 1 from The Effects of the Atmospheric Pressure on Evaporation Effect Of Pressure On Evaporation Calculate vapor density using vapor pressure. Calculate humidity and dew point. this process, called vaporization or evaporation, generates a vapor pressure above the liquid. the evaporation rate of a substance is positively associated with the difference between vapor pressure and. explain the relationship between vapor pressure of water and the capacity of air to hold water vapor.. Effect Of Pressure On Evaporation.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It Effect Of Pressure On Evaporation evaporation of a liquid body into a gas phase (air, in the present discussion) is spontaneous if the gas. Calculate vapor density using vapor pressure. Molecules in the gas phase can collide with the liquid surface and reenter the liquid via condensation. the evaporation rate of a substance is positively associated with the difference between vapor pressure and.. Effect Of Pressure On Evaporation.
From www.youtube.com
Matter In Our Surrounding Class 9 Science Change in Pressure on Effect Of Pressure On Evaporation according to the classical scatterplot, the relationship between pressure and evaporation was not strong. explain the relationship between vapor pressure of water and the capacity of air to hold water vapor. Calculate vapor density using vapor pressure. the main factors are air and water temperature, relative humidity, wind velocity, surface area, atmospheric. Calculate humidity and dew point.. Effect Of Pressure On Evaporation.
From chem.libretexts.org
Chapter 11.4 Vapor Pressure Chemistry LibreTexts Effect Of Pressure On Evaporation this process, called vaporization or evaporation, generates a vapor pressure above the liquid. the evaporation rate of a substance is positively associated with the difference between vapor pressure and. explain the relationship between vapor pressure of water and the capacity of air to hold water vapor. according to the classical scatterplot, the relationship between pressure and. Effect Of Pressure On Evaporation.