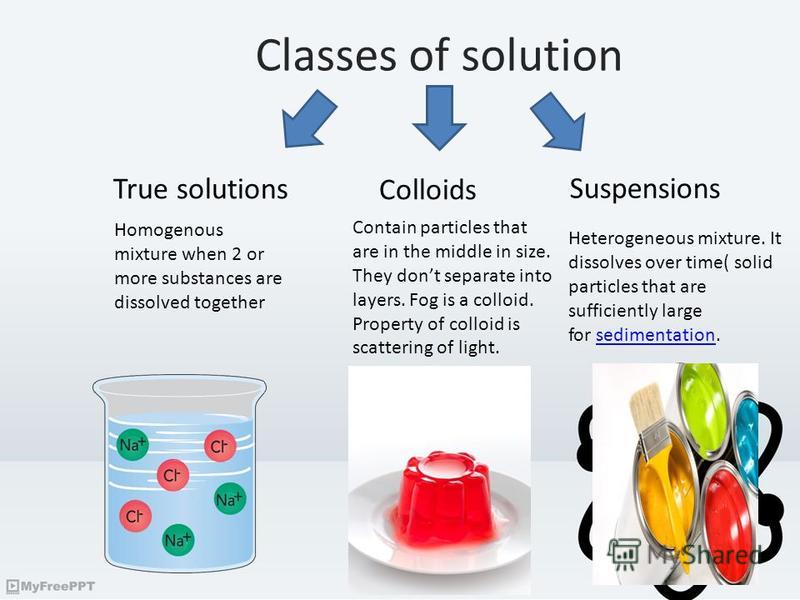
Suspension Vs Colloid Examples . Colloid particles are much smaller than suspension. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and. On the other hand, a suspension is a heterogeneous mixture where solid. the main difference between colloid and suspension lies in the size of particles. A solution is a homogeneous mixture of one or more substances dissolved in another. examples of colloids include milk, gelatin, and fog. here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. a suspension is a heterogeneous mixture in which some of the particles settle out of the mixture upon.
from www.myshared.ru
examples of colloids include milk, gelatin, and fog. a suspension is a heterogeneous mixture in which some of the particles settle out of the mixture upon. the main difference between colloid and suspension lies in the size of particles. On the other hand, a suspension is a heterogeneous mixture where solid. A solution is a homogeneous mixture of one or more substances dissolved in another. Colloid particles are much smaller than suspension. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and. here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each.
Презентация на тему "Colloidal systems. Classes of solution True
Suspension Vs Colloid Examples Colloid particles are much smaller than suspension. examples of colloids include milk, gelatin, and fog. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and. Colloid particles are much smaller than suspension. On the other hand, a suspension is a heterogeneous mixture where solid. a suspension is a heterogeneous mixture in which some of the particles settle out of the mixture upon. here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. A solution is a homogeneous mixture of one or more substances dissolved in another. the main difference between colloid and suspension lies in the size of particles.
From www.stepbystep.com
Difference Between Suspension and Colloid Suspension Vs Colloid Examples suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and. a suspension is a heterogeneous mixture in which some of the particles settle out of the mixture upon. Colloid particles are much smaller than suspension. examples of colloids include milk, gelatin, and fog. A solution is. Suspension Vs Colloid Examples.
From www.differencebetween.com
Difference Between Suspension and Colloid Compare the Difference Suspension Vs Colloid Examples On the other hand, a suspension is a heterogeneous mixture where solid. Colloid particles are much smaller than suspension. examples of colloids include milk, gelatin, and fog. A solution is a homogeneous mixture of one or more substances dissolved in another. a suspension is a heterogeneous mixture in which some of the particles settle out of the mixture. Suspension Vs Colloid Examples.
From 88guru.com
True solution, Suspension, Colloidal Solutions 88Guru Suspension Vs Colloid Examples examples of colloids include milk, gelatin, and fog. On the other hand, a suspension is a heterogeneous mixture where solid. here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. Colloid particles are much smaller than suspension. the main difference between colloid and suspension lies in the size. Suspension Vs Colloid Examples.
From brainly.in
How are colloids and suspensions different from solutions ? Brainly.in Suspension Vs Colloid Examples Colloid particles are much smaller than suspension. A solution is a homogeneous mixture of one or more substances dissolved in another. here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. examples of colloids include milk, gelatin, and fog. the main difference between colloid and suspension lies in. Suspension Vs Colloid Examples.
From hxegkfjyj.blob.core.windows.net
Is Paint A Solution Colloid Or Suspension at Ida Stites blog Suspension Vs Colloid Examples here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. On the other hand, a suspension is a heterogeneous mixture where solid. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and. the main difference between colloid. Suspension Vs Colloid Examples.
From pt.slideshare.net
Solutions, suspensions, and colloids Suspension Vs Colloid Examples A solution is a homogeneous mixture of one or more substances dissolved in another. the main difference between colloid and suspension lies in the size of particles. On the other hand, a suspension is a heterogeneous mixture where solid. here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each.. Suspension Vs Colloid Examples.
From www.e-streetlight.com
Solutions Colloids And Suspensions Worksheet E Street Light Suspension Vs Colloid Examples suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and. examples of colloids include milk, gelatin, and fog. the main difference between colloid and suspension lies in the size of particles. here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry,. Suspension Vs Colloid Examples.
From www.youtube.com
Solution Suspension Colloid YouTube Suspension Vs Colloid Examples A solution is a homogeneous mixture of one or more substances dissolved in another. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and. here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. the main difference. Suspension Vs Colloid Examples.
From www.slideserve.com
PPT Suspensions and Colloids PowerPoint Presentation, free download Suspension Vs Colloid Examples a suspension is a heterogeneous mixture in which some of the particles settle out of the mixture upon. Colloid particles are much smaller than suspension. A solution is a homogeneous mixture of one or more substances dissolved in another. here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each.. Suspension Vs Colloid Examples.
From www.myshared.ru
Презентация на тему "Colloidal systems. Classes of solution True Suspension Vs Colloid Examples here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. a suspension is a heterogeneous mixture in which some of the particles settle out of the mixture upon. A solution is a homogeneous mixture of one or more substances dissolved in another. On the other hand, a suspension is. Suspension Vs Colloid Examples.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Suspension Vs Colloid Examples a suspension is a heterogeneous mixture in which some of the particles settle out of the mixture upon. A solution is a homogeneous mixture of one or more substances dissolved in another. here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. the main difference between colloid and. Suspension Vs Colloid Examples.
From dxolbrjjh.blob.core.windows.net
Suspension Colloid Define at Howard Barber blog Suspension Vs Colloid Examples examples of colloids include milk, gelatin, and fog. a suspension is a heterogeneous mixture in which some of the particles settle out of the mixture upon. On the other hand, a suspension is a heterogeneous mixture where solid. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of. Suspension Vs Colloid Examples.
From www.studypage.in
True Solution, Colloidal Solution and Suspension Study Page Suspension Vs Colloid Examples a suspension is a heterogeneous mixture in which some of the particles settle out of the mixture upon. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and. here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of. Suspension Vs Colloid Examples.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Suspension Vs Colloid Examples A solution is a homogeneous mixture of one or more substances dissolved in another. On the other hand, a suspension is a heterogeneous mixture where solid. examples of colloids include milk, gelatin, and fog. here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. Colloid particles are much smaller. Suspension Vs Colloid Examples.
From exouqcyfe.blob.core.windows.net
What Is Colloidal Suspension In Chemistry at Robert Lemond blog Suspension Vs Colloid Examples here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. On the other hand, a suspension is a heterogeneous mixture where solid. a suspension is a heterogeneous mixture in which some of the particles settle out of the mixture upon. examples of colloids include milk, gelatin, and fog.. Suspension Vs Colloid Examples.
From www.slideshare.net
Solutions, suspensions, and colloids Suspension Vs Colloid Examples On the other hand, a suspension is a heterogeneous mixture where solid. the main difference between colloid and suspension lies in the size of particles. A solution is a homogeneous mixture of one or more substances dissolved in another. Colloid particles are much smaller than suspension. here is how to distinguish among solutions, suspensions, colloids, and other dispersions. Suspension Vs Colloid Examples.
From exoblfilw.blob.core.windows.net
Colloid Definition And Example at Benjamin Clift blog Suspension Vs Colloid Examples the main difference between colloid and suspension lies in the size of particles. Colloid particles are much smaller than suspension. On the other hand, a suspension is a heterogeneous mixture where solid. a suspension is a heterogeneous mixture in which some of the particles settle out of the mixture upon. A solution is a homogeneous mixture of one. Suspension Vs Colloid Examples.
From www.slideserve.com
PPT Solution, Suspensions and Colloids PowerPoint Presentation, free Suspension Vs Colloid Examples A solution is a homogeneous mixture of one or more substances dissolved in another. a suspension is a heterogeneous mixture in which some of the particles settle out of the mixture upon. On the other hand, a suspension is a heterogeneous mixture where solid. suspensions and colloids are two common types of mixtures whose properties are in many. Suspension Vs Colloid Examples.
From www.majordifferences.com
Difference Between True Solutions, Colloidal solution and Suspension Suspension Vs Colloid Examples Colloid particles are much smaller than suspension. the main difference between colloid and suspension lies in the size of particles. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and. here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with. Suspension Vs Colloid Examples.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Suspension Vs Colloid Examples a suspension is a heterogeneous mixture in which some of the particles settle out of the mixture upon. On the other hand, a suspension is a heterogeneous mixture where solid. examples of colloids include milk, gelatin, and fog. here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each.. Suspension Vs Colloid Examples.
From www.vecteezy.com
True Solution, Colloid solution and Suspension three different types of Suspension Vs Colloid Examples a suspension is a heterogeneous mixture in which some of the particles settle out of the mixture upon. the main difference between colloid and suspension lies in the size of particles. here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. examples of colloids include milk, gelatin,. Suspension Vs Colloid Examples.
From pediaa.com
Difference Between Colloid and Suspension Definition, Properties Suspension Vs Colloid Examples A solution is a homogeneous mixture of one or more substances dissolved in another. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and. On the other hand, a suspension is a heterogeneous mixture where solid. the main difference between colloid and suspension lies in the size. Suspension Vs Colloid Examples.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Suspension Vs Colloid Examples Colloid particles are much smaller than suspension. A solution is a homogeneous mixture of one or more substances dissolved in another. examples of colloids include milk, gelatin, and fog. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and. a suspension is a heterogeneous mixture in. Suspension Vs Colloid Examples.
From www.youtube.com
Solution, Suspension and Colloid aumsum kids education science Suspension Vs Colloid Examples a suspension is a heterogeneous mixture in which some of the particles settle out of the mixture upon. Colloid particles are much smaller than suspension. examples of colloids include milk, gelatin, and fog. here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. the main difference between. Suspension Vs Colloid Examples.
From www.slideshare.net
Unit 2, Lesson 2.5 Suspensions and Colloids Suspension Vs Colloid Examples examples of colloids include milk, gelatin, and fog. A solution is a homogeneous mixture of one or more substances dissolved in another. here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. the main difference between colloid and suspension lies in the size of particles. suspensions and. Suspension Vs Colloid Examples.
From www.youtube.com
Colloids, Solutions & Suspensions YouTube Suspension Vs Colloid Examples Colloid particles are much smaller than suspension. the main difference between colloid and suspension lies in the size of particles. A solution is a homogeneous mixture of one or more substances dissolved in another. here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. suspensions and colloids are. Suspension Vs Colloid Examples.
From www.youtube.com
TRUE SOLUTION COLLOID SUSPENSIONS 10 major differences. YouTube Suspension Vs Colloid Examples here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. the main difference between colloid and suspension lies in the size of particles. a suspension is a heterogeneous mixture in which some of the particles settle out of the mixture upon. A solution is a homogeneous mixture of. Suspension Vs Colloid Examples.
From quizizz.com
Solution, Colloid and Suspension Science Quizizz Suspension Vs Colloid Examples Colloid particles are much smaller than suspension. here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and. On the other hand, a suspension is a heterogeneous mixture where. Suspension Vs Colloid Examples.
From plantlet.org
Mixture Types Solution, Suspension, Colloids & Others Plantlet Suspension Vs Colloid Examples A solution is a homogeneous mixture of one or more substances dissolved in another. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and. a suspension is a heterogeneous mixture in which some of the particles settle out of the mixture upon. here is how to. Suspension Vs Colloid Examples.
From www.youtube.com
Solutions Suspensions and Colloids Part 1/1 English Class 9 YouTube Suspension Vs Colloid Examples On the other hand, a suspension is a heterogeneous mixture where solid. a suspension is a heterogeneous mixture in which some of the particles settle out of the mixture upon. A solution is a homogeneous mixture of one or more substances dissolved in another. examples of colloids include milk, gelatin, and fog. suspensions and colloids are two. Suspension Vs Colloid Examples.
From studylib.net
Solutions, Colloids, Suspension Suspension Vs Colloid Examples A solution is a homogeneous mixture of one or more substances dissolved in another. examples of colloids include milk, gelatin, and fog. the main difference between colloid and suspension lies in the size of particles. a suspension is a heterogeneous mixture in which some of the particles settle out of the mixture upon. On the other hand,. Suspension Vs Colloid Examples.
From in.pinterest.com
Solutions, suspensions, and colloids Suspension mixture, Body diagram Suspension Vs Colloid Examples suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and. Colloid particles are much smaller than suspension. the main difference between colloid and suspension lies in the size of particles. A solution is a homogeneous mixture of one or more substances dissolved in another. examples of. Suspension Vs Colloid Examples.
From giorttbfg.blob.core.windows.net
Solution Vs Dispersion at Douglas Smith blog Suspension Vs Colloid Examples A solution is a homogeneous mixture of one or more substances dissolved in another. here is how to distinguish among solutions, suspensions, colloids, and other dispersions in chemistry, along with examples of each. examples of colloids include milk, gelatin, and fog. Colloid particles are much smaller than suspension. suspensions and colloids are two common types of mixtures. Suspension Vs Colloid Examples.
From byjus.com
Suspensions (Chemistry) Definition, Properties, Examples with Videos Suspension Vs Colloid Examples A solution is a homogeneous mixture of one or more substances dissolved in another. examples of colloids include milk, gelatin, and fog. suspensions and colloids are two common types of mixtures whose properties are in many ways intermediate between those of homogeneous and. a suspension is a heterogeneous mixture in which some of the particles settle out. Suspension Vs Colloid Examples.
From www.geeksforgeeks.org
Suspension(Chemistry) Definition, Properties, Examples, and FAQs Suspension Vs Colloid Examples examples of colloids include milk, gelatin, and fog. the main difference between colloid and suspension lies in the size of particles. On the other hand, a suspension is a heterogeneous mixture where solid. Colloid particles are much smaller than suspension. A solution is a homogeneous mixture of one or more substances dissolved in another. a suspension is. Suspension Vs Colloid Examples.