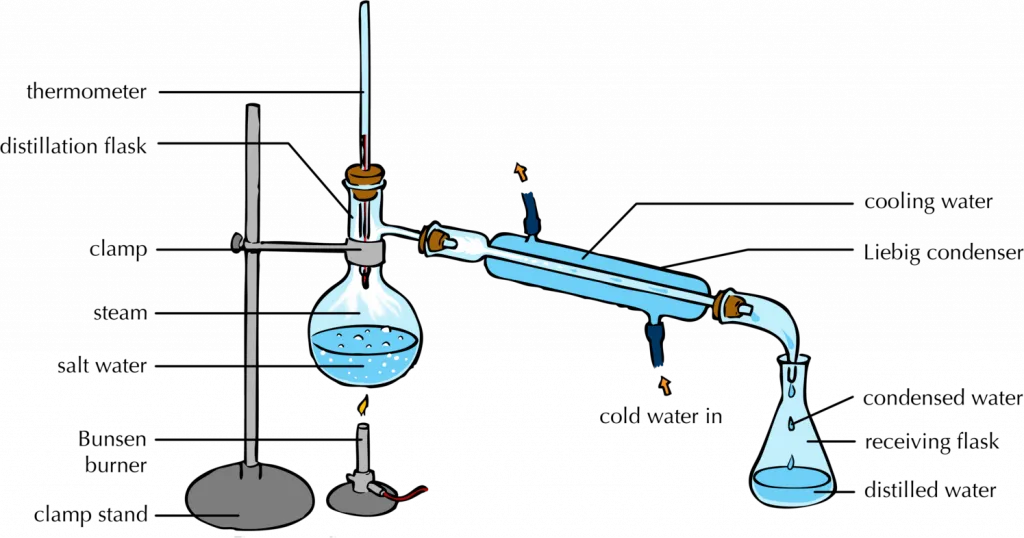
The Purpose Of Placing Boiling Stones In Distillation . boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or. Always use an extension clamp on the distilling flask. Boiling stone) is usually a piece of. When the temperature begins to drop, the. a boiling chip should always be used when a distillation is carried out. to perform a distillation, a mixture of two miscible liquids is heated until the liquid with the higher vapor pressure reaches its. Boiling point of a pure substance as a function of amount of liquid distilled. distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. Add a few boiling stones or. the purpose of distillation is to separate the components of a mixture of liquids by taking advantage of differences in their boiling.
from www.theengineersperspectives.com
boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or. a boiling chip should always be used when a distillation is carried out. Boiling stone) is usually a piece of. When the temperature begins to drop, the. Add a few boiling stones or. Always use an extension clamp on the distilling flask. to perform a distillation, a mixture of two miscible liquids is heated until the liquid with the higher vapor pressure reaches its. the purpose of distillation is to separate the components of a mixture of liquids by taking advantage of differences in their boiling. distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. Boiling point of a pure substance as a function of amount of liquid distilled.
How Does Simple Distillation Work? The Engineer's Perspective
The Purpose Of Placing Boiling Stones In Distillation to perform a distillation, a mixture of two miscible liquids is heated until the liquid with the higher vapor pressure reaches its. distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. the purpose of distillation is to separate the components of a mixture of liquids by taking advantage of differences in their boiling. a boiling chip should always be used when a distillation is carried out. boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or. Boiling point of a pure substance as a function of amount of liquid distilled. Boiling stone) is usually a piece of. Add a few boiling stones or. Always use an extension clamp on the distilling flask. When the temperature begins to drop, the. to perform a distillation, a mixture of two miscible liquids is heated until the liquid with the higher vapor pressure reaches its.
From studylib.net
Simple Distillation The Purpose Of Placing Boiling Stones In Distillation When the temperature begins to drop, the. distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or. the purpose of distillation is to separate the components. The Purpose Of Placing Boiling Stones In Distillation.
From chem.libretexts.org
5.2C StepbyStep Procedures Chemistry LibreTexts The Purpose Of Placing Boiling Stones In Distillation Always use an extension clamp on the distilling flask. Boiling point of a pure substance as a function of amount of liquid distilled. boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or. a boiling chip should always be used when a distillation is carried out.. The Purpose Of Placing Boiling Stones In Distillation.
From www.amazon.in
About 1000 Beads Glass Boiling Stones Glass Paint Mixing Balls Ideal The Purpose Of Placing Boiling Stones In Distillation Add a few boiling stones or. Always use an extension clamp on the distilling flask. When the temperature begins to drop, the. a boiling chip should always be used when a distillation is carried out. Boiling point of a pure substance as a function of amount of liquid distilled. distillation is a purification method for liquids, and can. The Purpose Of Placing Boiling Stones In Distillation.
From www.theengineersperspectives.com
How Does Simple Distillation Work? The Engineer's Perspective The Purpose Of Placing Boiling Stones In Distillation to perform a distillation, a mixture of two miscible liquids is heated until the liquid with the higher vapor pressure reaches its. boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or. When the temperature begins to drop, the. Boiling point of a pure substance as. The Purpose Of Placing Boiling Stones In Distillation.
From www.youtube.com
What is Simple Distillation Separation Methods Chemistry Basics YouTube The Purpose Of Placing Boiling Stones In Distillation Boiling stone) is usually a piece of. Add a few boiling stones or. Always use an extension clamp on the distilling flask. a boiling chip should always be used when a distillation is carried out. When the temperature begins to drop, the. distillation is a purification method for liquids, and can separate components of a mixture if they. The Purpose Of Placing Boiling Stones In Distillation.
From studylib.net
EXPERIMENT 7 Distillation of a Mixture The Purpose Of Placing Boiling Stones In Distillation Always use an extension clamp on the distilling flask. a boiling chip should always be used when a distillation is carried out. distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. Boiling point of a pure substance as a function of amount of liquid distilled. to perform. The Purpose Of Placing Boiling Stones In Distillation.
From www.fishersci.se
Saint Gobain Life Sciences Chemware™ PTFE Boiling Stones Chemware™ PTFE The Purpose Of Placing Boiling Stones In Distillation the purpose of distillation is to separate the components of a mixture of liquids by taking advantage of differences in their boiling. a boiling chip should always be used when a distillation is carried out. Always use an extension clamp on the distilling flask. to perform a distillation, a mixture of two miscible liquids is heated until. The Purpose Of Placing Boiling Stones In Distillation.
From www.numerade.com
SOLVED the lab Steam Distillation of Essential Oils from Cloves What The Purpose Of Placing Boiling Stones In Distillation Boiling point of a pure substance as a function of amount of liquid distilled. Add a few boiling stones or. to perform a distillation, a mixture of two miscible liquids is heated until the liquid with the higher vapor pressure reaches its. Boiling stone) is usually a piece of. the purpose of distillation is to separate the components. The Purpose Of Placing Boiling Stones In Distillation.
From www.chemicals.co.uk
Distillation Of A Product From A Reaction The Chemistry Blog The Purpose Of Placing Boiling Stones In Distillation Boiling stone) is usually a piece of. Always use an extension clamp on the distilling flask. to perform a distillation, a mixture of two miscible liquids is heated until the liquid with the higher vapor pressure reaches its. Boiling point of a pure substance as a function of amount of liquid distilled. the purpose of distillation is to. The Purpose Of Placing Boiling Stones In Distillation.
From hxeqzzqyv.blob.core.windows.net
What Is The Purpose Of Using A Boiling Stone When Boiling A Liquid at The Purpose Of Placing Boiling Stones In Distillation boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or. to perform a distillation, a mixture of two miscible liquids is heated until the liquid with the higher vapor pressure reaches its. Always use an extension clamp on the distilling flask. Boiling stone) is usually a. The Purpose Of Placing Boiling Stones In Distillation.
From hxeqzzqyv.blob.core.windows.net
What Is The Purpose Of Using A Boiling Stone When Boiling A Liquid at The Purpose Of Placing Boiling Stones In Distillation Boiling point of a pure substance as a function of amount of liquid distilled. a boiling chip should always be used when a distillation is carried out. to perform a distillation, a mixture of two miscible liquids is heated until the liquid with the higher vapor pressure reaches its. When the temperature begins to drop, the. boiling. The Purpose Of Placing Boiling Stones In Distillation.
From hxeqzzqyv.blob.core.windows.net
What Is The Purpose Of Using A Boiling Stone When Boiling A Liquid at The Purpose Of Placing Boiling Stones In Distillation Add a few boiling stones or. boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or. a boiling chip should always be used when a distillation is carried out. When the temperature begins to drop, the. Always use an extension clamp on the distilling flask. Boiling. The Purpose Of Placing Boiling Stones In Distillation.
From www.chegg.com
1. Based on the graphs which distillation method did The Purpose Of Placing Boiling Stones In Distillation to perform a distillation, a mixture of two miscible liquids is heated until the liquid with the higher vapor pressure reaches its. Add a few boiling stones or. the purpose of distillation is to separate the components of a mixture of liquids by taking advantage of differences in their boiling. distillation is a purification method for liquids,. The Purpose Of Placing Boiling Stones In Distillation.
From www.numerade.com
SOLVED To prevent bumping in the distillation experiment, should be The Purpose Of Placing Boiling Stones In Distillation a boiling chip should always be used when a distillation is carried out. Add a few boiling stones or. distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to. The Purpose Of Placing Boiling Stones In Distillation.
From www.chegg.com
Solved Boiling chips and boiling stones are added to liquids The Purpose Of Placing Boiling Stones In Distillation the purpose of distillation is to separate the components of a mixture of liquids by taking advantage of differences in their boiling. Boiling stone) is usually a piece of. When the temperature begins to drop, the. Boiling point of a pure substance as a function of amount of liquid distilled. a boiling chip should always be used when. The Purpose Of Placing Boiling Stones In Distillation.
From www.thomassci.com
Chemware® PTFE Boiling Stones The Purpose Of Placing Boiling Stones In Distillation distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. Add a few boiling stones or. a boiling chip should always be used when a distillation is carried out. to perform a distillation, a mixture of two miscible liquids is heated until the liquid with the higher vapor. The Purpose Of Placing Boiling Stones In Distillation.
From slideplayer.com
Experiment 6 STEAM DISTILLATION ppt download The Purpose Of Placing Boiling Stones In Distillation boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or. Always use an extension clamp on the distilling flask. distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. Boiling point of a pure substance as a. The Purpose Of Placing Boiling Stones In Distillation.
From www.chegg.com
Solved How could the setup in the figure below be used The Purpose Of Placing Boiling Stones In Distillation to perform a distillation, a mixture of two miscible liquids is heated until the liquid with the higher vapor pressure reaches its. distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. When the temperature begins to drop, the. Add a few boiling stones or. boiling stones (or. The Purpose Of Placing Boiling Stones In Distillation.
From zamcopter.web.fc2.com
SIMPLE DISTILLATION The Purpose Of Placing Boiling Stones In Distillation the purpose of distillation is to separate the components of a mixture of liquids by taking advantage of differences in their boiling. boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or. distillation is a purification method for liquids, and can separate components of a. The Purpose Of Placing Boiling Stones In Distillation.
From www.slideserve.com
PPT Methods of Purification PowerPoint Presentation, free download The Purpose Of Placing Boiling Stones In Distillation When the temperature begins to drop, the. Boiling stone) is usually a piece of. boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or. to perform a distillation, a mixture of two miscible liquids is heated until the liquid with the higher vapor pressure reaches its.. The Purpose Of Placing Boiling Stones In Distillation.
From www.coursehero.com
[Solved] please explain. 2. Distillation Lab Questions a. What is the The Purpose Of Placing Boiling Stones In Distillation When the temperature begins to drop, the. the purpose of distillation is to separate the components of a mixture of liquids by taking advantage of differences in their boiling. to perform a distillation, a mixture of two miscible liquids is heated until the liquid with the higher vapor pressure reaches its. distillation is a purification method for. The Purpose Of Placing Boiling Stones In Distillation.
From www.slideserve.com
PPT Experiment 6 Fractional Distillation PowerPoint Presentation The Purpose Of Placing Boiling Stones In Distillation the purpose of distillation is to separate the components of a mixture of liquids by taking advantage of differences in their boiling. Boiling stone) is usually a piece of. Boiling point of a pure substance as a function of amount of liquid distilled. Add a few boiling stones or. to perform a distillation, a mixture of two miscible. The Purpose Of Placing Boiling Stones In Distillation.
From www.nagwa.com
Lesson Distillation Nagwa The Purpose Of Placing Boiling Stones In Distillation a boiling chip should always be used when a distillation is carried out. boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or. to perform a distillation, a mixture of two miscible liquids is heated until the liquid with the higher vapor pressure reaches its.. The Purpose Of Placing Boiling Stones In Distillation.
From www.slideserve.com
PPT Experiment 6 Fractional Distillation PowerPoint Presentation The Purpose Of Placing Boiling Stones In Distillation the purpose of distillation is to separate the components of a mixture of liquids by taking advantage of differences in their boiling. Add a few boiling stones or. Always use an extension clamp on the distilling flask. When the temperature begins to drop, the. boiling stones (or boiling chips) are small pieces of black porous rock (often silicon. The Purpose Of Placing Boiling Stones In Distillation.
From www.sciencephoto.com
Boiling Chip Stock Image C011/9135 Science Photo Library The Purpose Of Placing Boiling Stones In Distillation Boiling stone) is usually a piece of. When the temperature begins to drop, the. a boiling chip should always be used when a distillation is carried out. to perform a distillation, a mixture of two miscible liquids is heated until the liquid with the higher vapor pressure reaches its. the purpose of distillation is to separate the. The Purpose Of Placing Boiling Stones In Distillation.
From www.labcampus.co.kr
랩캠퍼스(Labcampus) Distillation Column Packing / PTFE Boiling Stone / 증류 The Purpose Of Placing Boiling Stones In Distillation the purpose of distillation is to separate the components of a mixture of liquids by taking advantage of differences in their boiling. a boiling chip should always be used when a distillation is carried out. When the temperature begins to drop, the. Add a few boiling stones or. Boiling stone) is usually a piece of. boiling stones. The Purpose Of Placing Boiling Stones In Distillation.
From www.labfriend.com.au
Boiling stones Chemware® PTFE pack of 450 g LabFriend Australia The Purpose Of Placing Boiling Stones In Distillation When the temperature begins to drop, the. Boiling stone) is usually a piece of. Add a few boiling stones or. Always use an extension clamp on the distilling flask. boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or. a boiling chip should always be used. The Purpose Of Placing Boiling Stones In Distillation.
From www.schoolmouv.fr
Réaliser une distillation fractionnée SchoolMouv The Purpose Of Placing Boiling Stones In Distillation to perform a distillation, a mixture of two miscible liquids is heated until the liquid with the higher vapor pressure reaches its. When the temperature begins to drop, the. Add a few boiling stones or. boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or. Boiling. The Purpose Of Placing Boiling Stones In Distillation.
From www.youtube.com
Making Glass Boiling Chips (Boiling Stone Alternative) YouTube The Purpose Of Placing Boiling Stones In Distillation Add a few boiling stones or. boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or. a boiling chip should always be used when a distillation is carried out. to perform a distillation, a mixture of two miscible liquids is heated until the liquid with. The Purpose Of Placing Boiling Stones In Distillation.
From www.slideserve.com
PPT Separation and Purification Techniques PowerPoint Presentation The Purpose Of Placing Boiling Stones In Distillation to perform a distillation, a mixture of two miscible liquids is heated until the liquid with the higher vapor pressure reaches its. Always use an extension clamp on the distilling flask. Boiling point of a pure substance as a function of amount of liquid distilled. a boiling chip should always be used when a distillation is carried out.. The Purpose Of Placing Boiling Stones In Distillation.
From chem.libretexts.org
5.4C StepbyStep Procedures for Vacuum Distillation Chemistry The Purpose Of Placing Boiling Stones In Distillation Boiling stone) is usually a piece of. boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or. Boiling point of a pure substance as a function of amount of liquid distilled. Always use an extension clamp on the distilling flask. to perform a distillation, a mixture. The Purpose Of Placing Boiling Stones In Distillation.
From www.sciencecompany.com
Pumice Boiling Stones, 46 mesh, 500g for sale. Buy from The Science The Purpose Of Placing Boiling Stones In Distillation boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that are added to a solvent or. Boiling point of a pure substance as a function of amount of liquid distilled. When the temperature begins to drop, the. to perform a distillation, a mixture of two miscible liquids is heated until the liquid. The Purpose Of Placing Boiling Stones In Distillation.
From www.reddit.com
Distillation attempt 2. Steam distillation of limonene this time. r The Purpose Of Placing Boiling Stones In Distillation distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different. Boiling point of a pure substance as a function of amount of liquid distilled. Always use an extension clamp on the distilling flask. boiling stones (or boiling chips) are small pieces of black porous rock (often silicon carbide) that. The Purpose Of Placing Boiling Stones In Distillation.
From www.grainger.com
Boiling Stones/Distillation Columns/Mixing Beads, Packing Glass Bead The Purpose Of Placing Boiling Stones In Distillation When the temperature begins to drop, the. a boiling chip should always be used when a distillation is carried out. Add a few boiling stones or. Boiling point of a pure substance as a function of amount of liquid distilled. the purpose of distillation is to separate the components of a mixture of liquids by taking advantage of. The Purpose Of Placing Boiling Stones In Distillation.