Pressure And Temperature And Volume Relationship . Relation between the pressure, volume, amount, and temperature of a gas under conditions derived by combination of the simple. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and temperature, for example), varying a third (such as pressure), and measuring the effect of the change on the fourth (in this case, volume). boyle's law states that at constant temperature the volume of a given mass of a dry gas is inversely proportional to its. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount. the volume of a given amount of gas is inversely proportional to its pressure when temperature is held.
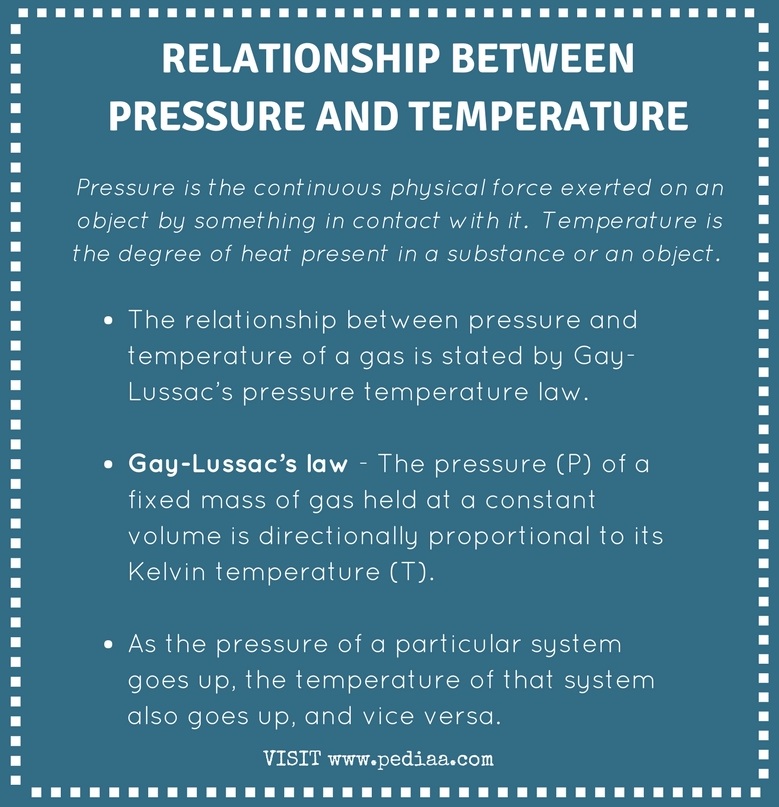
from pediaa.com
boyle's law states that at constant temperature the volume of a given mass of a dry gas is inversely proportional to its. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and temperature, for example), varying a third (such as pressure), and measuring the effect of the change on the fourth (in this case, volume). the volume of a given amount of gas is inversely proportional to its pressure when temperature is held. Relation between the pressure, volume, amount, and temperature of a gas under conditions derived by combination of the simple. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount.
Relationship Between Pressure and Temperature
Pressure And Temperature And Volume Relationship early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount. the volume of a given amount of gas is inversely proportional to its pressure when temperature is held. boyle's law states that at constant temperature the volume of a given mass of a dry gas is inversely proportional to its. Relation between the pressure, volume, amount, and temperature of a gas under conditions derived by combination of the simple. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and temperature, for example), varying a third (such as pressure), and measuring the effect of the change on the fourth (in this case, volume). early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount.
From www.youtube.com
Pressure, Volume and Temperature Relationships Chemistry Tutorial Pressure And Temperature And Volume Relationship early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount. Relation between the pressure, volume, amount, and temperature of a gas under conditions derived by combination of the simple. the volume of a given amount of gas is inversely proportional to its pressure when temperature is held. . Pressure And Temperature And Volume Relationship.
From www.slideserve.com
PPT Pressure, temperature and volume relationships (w/ a constant Pressure And Temperature And Volume Relationship the volume of a given amount of gas is inversely proportional to its pressure when temperature is held. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and temperature, for example), varying a third (such as pressure),. Pressure And Temperature And Volume Relationship.
From www.physics.brocku.ca
PPLATO FLAP PHYS 7.2 Temperature, pressure and the ideal gas laws Pressure And Temperature And Volume Relationship Relation between the pressure, volume, amount, and temperature of a gas under conditions derived by combination of the simple. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and temperature, for example), varying a third (such as pressure),. Pressure And Temperature And Volume Relationship.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Pressure And Temperature And Volume Relationship early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount. boyle's law states that at constant temperature the volume of a given mass of a dry gas is inversely proportional to its. Relation between the pressure, volume, amount, and temperature of a gas under conditions derived by combination. Pressure And Temperature And Volume Relationship.
From opentextbc.ca
9.2 Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Pressure And Temperature And Volume Relationship early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount. Relation between the pressure, volume, amount, and temperature of a gas under conditions derived by combination of the simple. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and. Pressure And Temperature And Volume Relationship.
From saylordotorg.github.io
Relationships among Pressure, Temperature, Volume, and Amount Pressure And Temperature And Volume Relationship early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and temperature, for example), varying a third (such as pressure), and measuring the effect of the change on the fourth (in this case, volume). boyle's law states that. Pressure And Temperature And Volume Relationship.
From www.markedbyteachers.com
Investigating the relationship between pressure, volume and temperature Pressure And Temperature And Volume Relationship the volume of a given amount of gas is inversely proportional to its pressure when temperature is held. Relation between the pressure, volume, amount, and temperature of a gas under conditions derived by combination of the simple. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount. . Pressure And Temperature And Volume Relationship.
From cartoondealer.com
Boyle's Law, Relationship Between Pressure And Volume Of Gas At Pressure And Temperature And Volume Relationship boyle's law states that at constant temperature the volume of a given mass of a dry gas is inversely proportional to its. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and temperature, for example), varying a. Pressure And Temperature And Volume Relationship.
From flatworldknowledge.lardbucket.org
Relationships among Pressure, Temperature, Volume, and Amount Pressure And Temperature And Volume Relationship boyle's law states that at constant temperature the volume of a given mass of a dry gas is inversely proportional to its. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount. the volume of a given amount of gas is inversely proportional to its pressure when. Pressure And Temperature And Volume Relationship.
From www.youtube.com
Relation between Pressure, Volume and Temperature in Adiabatic Process Pressure And Temperature And Volume Relationship the volume of a given amount of gas is inversely proportional to its pressure when temperature is held. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount. boyle's law states that at constant temperature the volume of a given mass of a dry gas is inversely. Pressure And Temperature And Volume Relationship.
From www.slideserve.com
PPT Pressure, Volume, Temperature The Gas Laws PowerPoint Pressure And Temperature And Volume Relationship early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and temperature, for example), varying a third (such as pressure), and measuring the effect of the change on the fourth (in this case, volume). the volume of a. Pressure And Temperature And Volume Relationship.
From www.youtube.com
Proof of Pressure, Volume and Temperature Ratio Adiabatic Process Pressure And Temperature And Volume Relationship Relation between the pressure, volume, amount, and temperature of a gas under conditions derived by combination of the simple. the volume of a given amount of gas is inversely proportional to its pressure when temperature is held. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n). Pressure And Temperature And Volume Relationship.
From flatworldknowledge.lardbucket.org
Relationships among Pressure, Temperature, Volume, and Amount Pressure And Temperature And Volume Relationship early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and temperature, for example), varying a third (such as pressure), and measuring the effect of the change on the fourth (in this case, volume). early scientists explored the. Pressure And Temperature And Volume Relationship.
From pediaa.com
Relationship Between Pressure and Temperature Pressure And Temperature And Volume Relationship the volume of a given amount of gas is inversely proportional to its pressure when temperature is held. Relation between the pressure, volume, amount, and temperature of a gas under conditions derived by combination of the simple. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n). Pressure And Temperature And Volume Relationship.
From alevelphysicsnotes.com
Mr Toogood Physics The Experimental gas laws Pressure And Temperature And Volume Relationship the volume of a given amount of gas is inversely proportional to its pressure when temperature is held. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount. Relation between the pressure, volume, amount, and temperature of a gas under conditions derived by combination of the simple. . Pressure And Temperature And Volume Relationship.
From www.youtube.com
1.4.6 Solve problems involving temperature, pressure and volume for an Pressure And Temperature And Volume Relationship Relation between the pressure, volume, amount, and temperature of a gas under conditions derived by combination of the simple. boyle's law states that at constant temperature the volume of a given mass of a dry gas is inversely proportional to its. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume. Pressure And Temperature And Volume Relationship.
From flatworldknowledge.lardbucket.org
Relationships among Pressure, Temperature, Volume, and Amount Pressure And Temperature And Volume Relationship the volume of a given amount of gas is inversely proportional to its pressure when temperature is held. boyle's law states that at constant temperature the volume of a given mass of a dry gas is inversely proportional to its. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume. Pressure And Temperature And Volume Relationship.
From engineerexcel.com
Pressure Temperature Graphs Explained EngineerExcel Pressure And Temperature And Volume Relationship early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and temperature, for example), varying a third (such as pressure), and measuring the effect of the change on the fourth (in this case, volume). early scientists explored the. Pressure And Temperature And Volume Relationship.
From guidewiringdietician.z5.web.core.windows.net
Equation Relating Pressure Volume And Temp Pressure And Temperature And Volume Relationship boyle's law states that at constant temperature the volume of a given mass of a dry gas is inversely proportional to its. Relation between the pressure, volume, amount, and temperature of a gas under conditions derived by combination of the simple. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume. Pressure And Temperature And Volume Relationship.
From socratic.org
Which graph shows the relationship between the temperature and volume Pressure And Temperature And Volume Relationship early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and temperature, for example), varying a third (such as pressure), and measuring the effect of the change on the fourth (in this case, volume). early scientists explored the. Pressure And Temperature And Volume Relationship.
From opentextbc.ca
9.2 Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Pressure And Temperature And Volume Relationship Relation between the pressure, volume, amount, and temperature of a gas under conditions derived by combination of the simple. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and temperature, for example), varying a third (such as pressure),. Pressure And Temperature And Volume Relationship.
From www.vrogue.co
Which Graph Shows The Relationship Between The Temper vrogue.co Pressure And Temperature And Volume Relationship early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and temperature, for example), varying a third (such as pressure), and measuring the effect of the change on the fourth (in this case, volume). early scientists explored the. Pressure And Temperature And Volume Relationship.
From www.dreamstime.com
Boyle S Law Showing the Pressure and Volume Relationship Stock Vector Pressure And Temperature And Volume Relationship Relation between the pressure, volume, amount, and temperature of a gas under conditions derived by combination of the simple. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and temperature, for example), varying a third (such as pressure),. Pressure And Temperature And Volume Relationship.
From stock.adobe.com
Boyle's Law, Relationship between pressure and volume of gas at Pressure And Temperature And Volume Relationship the volume of a given amount of gas is inversely proportional to its pressure when temperature is held. Relation between the pressure, volume, amount, and temperature of a gas under conditions derived by combination of the simple. boyle's law states that at constant temperature the volume of a given mass of a dry gas is inversely proportional to. Pressure And Temperature And Volume Relationship.
From chem.libretexts.org
6.3 Relationships among Pressure, Temperature, Volume, and Amount Pressure And Temperature And Volume Relationship Relation between the pressure, volume, amount, and temperature of a gas under conditions derived by combination of the simple. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount. boyle's law states that at constant temperature the volume of a given mass of a dry gas is inversely. Pressure And Temperature And Volume Relationship.
From www.vecteezy.com
Boyle's Law, Relationship between pressure and volume of gas at Pressure And Temperature And Volume Relationship boyle's law states that at constant temperature the volume of a given mass of a dry gas is inversely proportional to its. Relation between the pressure, volume, amount, and temperature of a gas under conditions derived by combination of the simple. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume. Pressure And Temperature And Volume Relationship.
From www.slideserve.com
PPT Pressure, Volume, Temperature The Gas Laws PowerPoint Pressure And Temperature And Volume Relationship boyle's law states that at constant temperature the volume of a given mass of a dry gas is inversely proportional to its. Relation between the pressure, volume, amount, and temperature of a gas under conditions derived by combination of the simple. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume. Pressure And Temperature And Volume Relationship.
From guidelibperplexing.z13.web.core.windows.net
Pressure Volume And Temperature Equation Pressure And Temperature And Volume Relationship Relation between the pressure, volume, amount, and temperature of a gas under conditions derived by combination of the simple. boyle's law states that at constant temperature the volume of a given mass of a dry gas is inversely proportional to its. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume. Pressure And Temperature And Volume Relationship.
From masterconceptsinchemistry.com
What’s the relationship between pressure and volume of gas? Core Pressure And Temperature And Volume Relationship early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount. boyle's law states that at constant temperature the volume of a given mass of a dry gas is inversely proportional to its. Relation between the pressure, volume, amount, and temperature of a gas under conditions derived by combination. Pressure And Temperature And Volume Relationship.
From courses.lumenlearning.com
Phase Changes Physics Pressure And Temperature And Volume Relationship boyle's law states that at constant temperature the volume of a given mass of a dry gas is inversely proportional to its. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and temperature, for example), varying a. Pressure And Temperature And Volume Relationship.
From www.youtube.com
Pressure, Volume, Temperature and Mole Relationships YouTube Pressure And Temperature And Volume Relationship early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and temperature, for example), varying a third (such as pressure), and measuring the effect of the change on the fourth (in this case, volume). boyle's law states that. Pressure And Temperature And Volume Relationship.
From slidesharenow.blogspot.com
Pressure And Volume Relation slideshare Pressure And Temperature And Volume Relationship early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount. Relation between the pressure, volume, amount, and temperature of a gas under conditions derived by combination of the simple. boyle's law states that at constant temperature the volume of a given mass of a dry gas is inversely. Pressure And Temperature And Volume Relationship.
From www.slideserve.com
PPT Collisions of Gas Particles PowerPoint Presentation, free Pressure And Temperature And Volume Relationship boyle's law states that at constant temperature the volume of a given mass of a dry gas is inversely proportional to its. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and temperature, for example), varying a. Pressure And Temperature And Volume Relationship.
From www.pinterest.co.uk
A mathematical derivation of the equations relating the pressure Pressure And Temperature And Volume Relationship the volume of a given amount of gas is inversely proportional to its pressure when temperature is held. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount (n) by holding two of the four variables constant (amount and temperature, for example), varying a third (such as pressure),. Pressure And Temperature And Volume Relationship.
From www.alamy.com
Charles's law. relationship between volume and temperature. Increasing Pressure And Temperature And Volume Relationship boyle's law states that at constant temperature the volume of a given mass of a dry gas is inversely proportional to its. early scientists explored the relationships among the pressure of a gas (p) and its temperature (t), volume (v), and amount. the volume of a given amount of gas is inversely proportional to its pressure when. Pressure And Temperature And Volume Relationship.