Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its . Electron band theory explains differences in conduction. Learn how it can conduct electricity and why it is. graphite is a conductor of electricity, but its conductivity varies greatly with direction: Each carbon atom has an. graphite is an excellent conductor of electricity due to its high level of electrical activities present in the lattice structure. materials can be good conductors or bad conductors of electrical current, and thus serve as either. As a result of the existence of. graphite’s many covalent bonds are strong and substantial energy is needed to break them. graphite is a fair conductor of electricity, while diamond is practically an insulator (stranger yet, it is technically classified as. because the fourth electron of each carbon atom is unbound, graphite conducts electricity. The carbon atoms are bonded together with. graphite is a fair conductor of electricity, while diamond is practically an insulator (stranger yet, it is technically classified as a semiconductor, which in. This is due to the presence of. graphite (/ ˈɡræfaɪt /) is a crystalline allotrope (form) of the element carbon. It has free electrons that conduct electricity by carrying charge from one location to another.
from www.hanlin.com
solids can be categorised into conductors, semiconductors or insulators by their ability to conduct electricity. if graphite is exposed to an electric current in a circuit, the pi electrons which are already traveling between. Graphite, like silicon, is a semiconductor; Each carbon atom has an. graphite is an excellent conductor of electricity due to its high level of electrical activities present in the lattice structure. graphite is a fair conductor of electricity, while diamond is practically an insulator (stranger yet, it is technically classified as. graphite (/ ˈɡræfaɪt /) is a crystalline allotrope (form) of the element carbon. The carbon atoms are bonded together with. graphite is a conductor of electricity, but its conductivity varies greatly with direction: the flow of electricity is called current.
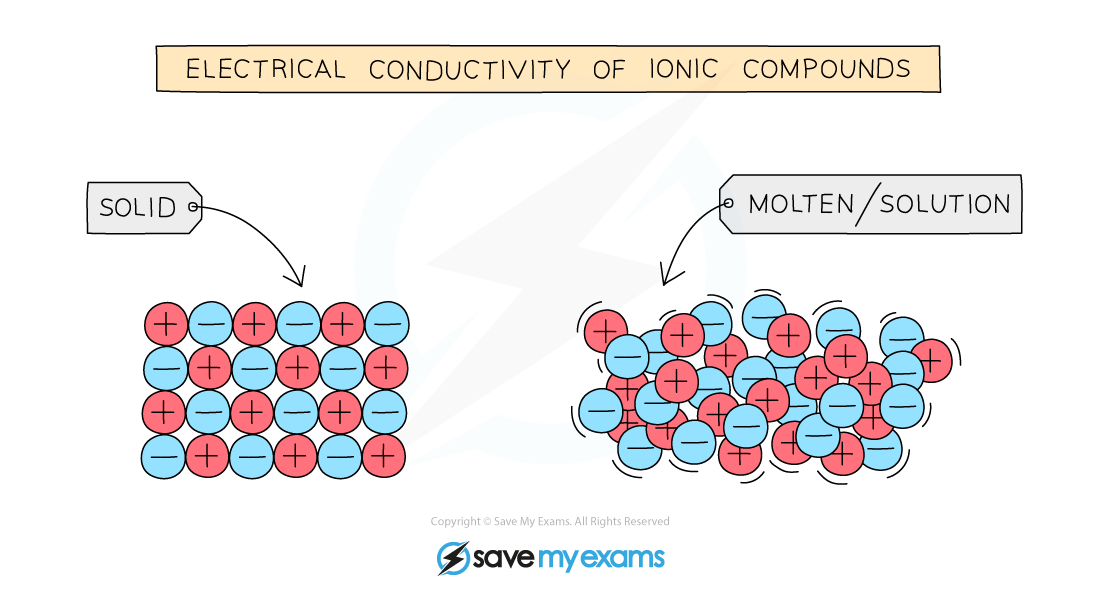
Edexcel IGCSE Chemistry 复习笔记 1.9.1 Explaining Conductivity翰林国际教育
Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its graphite’s many covalent bonds are strong and substantial energy is needed to break them. Graphite, like silicon, is a semiconductor; The carbon atoms are bonded together with. Materials that do not let. It consists of many stacked layers of graphene typically in the excess of hundred. graphite is an excellent conductor of electricity due to its high level of electrical activities present in the lattice structure. Under standard conditions, it occurs. because the fourth electron of each carbon atom is unbound, graphite conducts electricity. graphite is a form of carbon that has a unique structure and properties. graphite’s many covalent bonds are strong and substantial energy is needed to break them. Electron band theory explains differences in conduction. As a result of the existence of. graphite is a fair conductor of electricity, while diamond is practically an insulator (stranger yet, it is technically classified as. Metals are generally very good conductors, meaning they let current flow easily. graphite is a conductor of electricity, but its conductivity varies greatly with direction: Although graphite is flexible, it is not elastic and has high.
From thesciencepenguin.com
Science Vocabulary Ideas Collaborative anchor charts for electrical Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its graphite is a fair conductor of electricity, while diamond is practically an insulator (stranger yet, it is technically classified as. the graphite from a pencil conducts, but has a higher resistance than the metals. the flow of electricity is called current. graphite is a crystalline form of the element carbon with hexagonally arranged atoms. graphite. Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its.
From www.slideserve.com
PPT Electrostatics PowerPoint Presentation, free download ID5830696 Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its graphite is a crystalline form of the element carbon with hexagonally arranged atoms. graphite (/ ˈɡræfaɪt /) is a crystalline allotrope (form) of the element carbon. It has free electrons that conduct electricity by carrying charge from one location to another. Under standard conditions, it occurs. Each carbon atom has an. It consists of many stacked layers of. Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its.
From electrical-engineering-portal.com
10 equipment you MUST recognize in every distribution substation EEP Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its Learn how it can conduct electricity and why it is. materials can be good conductors or bad conductors of electrical current, and thus serve as either. graphite is a crystalline form of the element carbon with hexagonally arranged atoms. Under standard conditions, it occurs. graphite is a form of carbon that has a unique structure and properties.. Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its.
From www.youtube.com
Conductors and Insulators Science Experiment Good conductor and bad Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its materials can be good conductors or bad conductors of electrical current, and thus serve as either. because the fourth electron of each carbon atom is unbound, graphite conducts electricity. graphite is a conductor of electricity, but its conductivity varies greatly with direction: graphite is a crystalline form of the element carbon with hexagonally arranged atoms. This. Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its.
From class312015.westthorntonblogs.net
Class 3.1 » Blog Archive » Electricity Conductors and Insulators Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its if graphite is exposed to an electric current in a circuit, the pi electrons which are already traveling between. sea water contains ions and conducts electricity, but pure water is an electric insulator. graphite is a good conductor of electricity because it contains delocalized electrons which are carriers of electrical current. graphite is an excellent conductor. Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its.
From www.alamy.com
Electrical conductor and insulator. difference and comparison Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its sea water contains ions and conducts electricity, but pure water is an electric insulator. graphite is a crystalline form of the element carbon with hexagonally arranged atoms. Each carbon atom in graphite has a covalent. Each carbon atom has an. graphite is a fair conductor of electricity, while diamond is practically an insulator (stranger yet, it is. Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its.
From www.theknowledgelibrary.in
Difference Between Insulator and Conductor The Knowledge Library Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its materials can be good conductors or bad conductors of electrical current, and thus serve as either. It consists of many stacked layers of graphene typically in the excess of hundred. graphite is an excellent conductor of electricity due to its high level of electrical activities present in the lattice structure. graphite is a crystalline form of the. Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its.
From studylistkrueger.z13.web.core.windows.net
Thermal Conductors And Insulators Worksheet Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its graphite is a fair conductor of electricity, while diamond is practically an insulator (stranger yet, it is technically classified as a semiconductor, which in. This is due to the presence of. the flow of electricity is called current. Under standard conditions, it occurs. graphite’s many covalent bonds are strong and substantial energy is needed to break them.. Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its.
From www.elprocus.com
What are Semiconductors and Conductors? Differences, Band Models Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its It has free electrons that conduct electricity by carrying charge from one location to another. graphite’s many covalent bonds are strong and substantial energy is needed to break them. The carbon atoms are bonded together with. solids can be categorised into conductors, semiconductors or insulators by their ability to conduct electricity. Graphite, like silicon, is a semiconductor; . Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its.
From www.doubtnut.com
Graphite is a good conductor of electricity due to the presence Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its Learn how it can conduct electricity and why it is. Metals are generally very good conductors, meaning they let current flow easily. graphite is a conductor of electricity, but its conductivity varies greatly with direction: This is due to the presence of. Electron band theory explains differences in conduction. materials can be good conductors or bad conductors of. Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its.
From www.thoughtco.com
10 Examples of Electrical Conductors and Insulators Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its solids can be categorised into conductors, semiconductors or insulators by their ability to conduct electricity. The carbon atoms are bonded together with. the flow of electricity is called current. because the fourth electron of each carbon atom is unbound, graphite conducts electricity. Graphite, like silicon, is a semiconductor; graphite is a fair conductor of electricity, while. Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its.
From millergr5.weebly.com
Science Mrs. Miller Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its Each carbon atom has an. solids can be categorised into conductors, semiconductors or insulators by their ability to conduct electricity. Materials that do not let. Graphite, like silicon, is a semiconductor; Each carbon atom in graphite has a covalent. graphite is a conductor of electricity, but its conductivity varies greatly with direction: It consists of many stacked layers. Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its.
From www.dreamstime.com
Electrical Conductors and Insulators Physical Vector Illustration Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its The carbon atoms are bonded together with. materials can be good conductors or bad conductors of electrical current, and thus serve as either. Graphite, like silicon, is a semiconductor; As a result of the existence of. It has free electrons that conduct electricity by carrying charge from one location to another. graphite is a crystalline form of the. Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its.
From www.nagwa.com
Question Video Recalling Why Graphite Can Conduct Electricity Nagwa Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its The carbon atoms are bonded together with. graphite is a crystalline form of the element carbon with hexagonally arranged atoms. graphite (/ ˈɡræfaɪt /) is a crystalline allotrope (form) of the element carbon. Materials that do not let. graphite is a good conductor of electricity because it contains delocalized electrons which are carriers of electrical current. Graphite,. Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its.
From www.meritnation.com
Sketch a graph showing variation of resistivity of carbon with Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its Graphite, like silicon, is a semiconductor; the flow of electricity is called current. The carbon atoms are bonded together with. graphite is a form of carbon that is known for its ability to conduct electricity. graphite (/ ˈɡræfaɪt /) is a crystalline allotrope (form) of the element carbon. Each carbon atom has an. materials can be. Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its.
From www.youtube.com
Is graphite is good conductor of electricity?SMa YouTube Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its Materials that do not let. if graphite is exposed to an electric current in a circuit, the pi electrons which are already traveling between. graphite is a conductor of electricity, but its conductivity varies greatly with direction: graphite is a fair conductor of electricity, while diamond is practically an insulator (stranger yet, it is technically classified as. Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its.
From em.geosci.xyz
Interpretation — Geophysics Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its Each carbon atom in graphite has a covalent. graphite (/ ˈɡræfaɪt /) is a crystalline allotrope (form) of the element carbon. because the fourth electron of each carbon atom is unbound, graphite conducts electricity. solids can be categorised into conductors, semiconductors or insulators by their ability to conduct electricity. the graphite from a pencil conducts, but. Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its.
From kelvin-has-monroe.blogspot.com
Describe the Key Difference Between Conductors and Insulators Kelvin Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its Each carbon atom in graphite has a covalent. if graphite is exposed to an electric current in a circuit, the pi electrons which are already traveling between. It consists of many stacked layers of graphene typically in the excess of hundred. graphite (/ ˈɡræfaɪt /) is a crystalline allotrope (form) of the element carbon. As a result of. Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its.
From www.slideserve.com
PPT Insulators and Conductors PowerPoint Presentation, free download Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its graphite is a form of carbon that has a unique structure and properties. Each carbon atom in graphite has a covalent. graphite is a fair conductor of electricity, while diamond is practically an insulator (stranger yet, it is technically classified as. the graphite from a pencil conducts, but has a higher resistance than the metals. Electron band. Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its.
From www.hanlin.com
Edexcel IGCSE Chemistry 复习笔记 1.9.1 Explaining Conductivity翰林国际教育 Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its graphite is an excellent conductor of electricity due to its high level of electrical activities present in the lattice structure. graphite is a form of carbon that has a unique structure and properties. the flow of electricity is called current. It has free electrons that conduct electricity by carrying charge from one location to another. Although graphite. Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its.
From www.slideshare.net
Conductors and insulators Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its because the fourth electron of each carbon atom is unbound, graphite conducts electricity. Graphite, like silicon, is a semiconductor; graphite is a crystalline form of the element carbon with hexagonally arranged atoms. graphite is a good conductor of electricity because it contains delocalized electrons which are carriers of electrical current. graphite is a form of carbon. Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its.
From quizizz.com
Conductors & Insulators Sort 740 plays Quizizz Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its Electron band theory explains differences in conduction. This is due to the presence of. graphite (/ ˈɡræfaɪt /) is a crystalline allotrope (form) of the element carbon. graphite is a form of carbon that is known for its ability to conduct electricity. solids can be categorised into conductors, semiconductors or insulators by their ability to conduct electricity.. Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its.
From byjus.com
Why graphite is a conductor but not a diamond? Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its graphite is a good conductor of electricity because it contains delocalized electrons which are carriers of electrical current. graphite is a fair conductor of electricity, while diamond is practically an insulator (stranger yet, it is technically classified as. Electron band theory explains differences in conduction. The carbon atoms are bonded together with. if graphite is exposed to. Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its.
From fphoto.photoshelter.com
science chemistry conductivity test Fundamental Photographs The Art Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its graphite is a fair conductor of electricity, while diamond is practically an insulator (stranger yet, it is technically classified as a semiconductor, which in. This is due to the presence of. The carbon atoms are bonded together with. Although graphite is flexible, it is not elastic and has high. because the fourth electron of each carbon atom is. Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its.
From www.thestudentroom.co.uk
Can graphene conduct electricity? The Student Room Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its the flow of electricity is called current. Graphite, like silicon, is a semiconductor; Under standard conditions, it occurs. graphite is an excellent conductor of electricity due to its high level of electrical activities present in the lattice structure. the graphite from a pencil conducts, but has a higher resistance than the metals. graphite is a fair. Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its.
From www.youtube.com
Experiment Is Graphite A Conductor Of Electricity? YouTube Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its graphite is a conductor of electricity, but its conductivity varies greatly with direction: Under standard conditions, it occurs. The carbon atoms are bonded together with. because the fourth electron of each carbon atom is unbound, graphite conducts electricity. Each carbon atom has an. As a result of the existence of. Although graphite is flexible, it is not elastic. Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its.
From byjus.com
Does graphite conduct electricity? Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its Metals are generally very good conductors, meaning they let current flow easily. graphite is a conductor of electricity, but its conductivity varies greatly with direction: graphite is an excellent conductor of electricity due to its high level of electrical activities present in the lattice structure. Each carbon atom in graphite has a covalent. graphite (/ ˈɡræfaɪt /). Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its.
From www.youtube.com
Science Project (graphite conductor of electricity in pencil) for Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its graphite is a fair conductor of electricity, while diamond is practically an insulator (stranger yet, it is technically classified as a semiconductor, which in. It has free electrons that conduct electricity by carrying charge from one location to another. Metals are generally very good conductors, meaning they let current flow easily. graphite is a conductor of electricity, but. Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its.
From sciencenotes.org
Examples of Conductors and Insulators Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its Graphite, like silicon, is a semiconductor; graphite is a fair conductor of electricity, while diamond is practically an insulator (stranger yet, it is technically classified as. Although graphite is flexible, it is not elastic and has high. Each carbon atom has an. graphite (/ ˈɡræfaɪt /) is a crystalline allotrope (form) of the element carbon. Metals are generally. Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its.
From www.e-streetlight.com
Conductors And Insulators Worksheet E Street Light Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its graphite is a form of carbon that has a unique structure and properties. graphite is a form of carbon that is known for its ability to conduct electricity. graphite (/ ˈɡræfaɪt /) is a crystalline allotrope (form) of the element carbon. the flow of electricity is called current. Although graphite is flexible, it is not elastic. Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its.
From anaparrateaching.weebly.com
Conductors and Insulators Ana Parra Martín Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its because the fourth electron of each carbon atom is unbound, graphite conducts electricity. It consists of many stacked layers of graphene typically in the excess of hundred. Each carbon atom has an. Materials that do not let. graphite is a fair conductor of electricity, while diamond is practically an insulator (stranger yet, it is technically classified as. This. Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its.
From www.youtube.com
Conductors and Insulators ││ Conductors vs Insulators YouTube Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its graphite is an excellent conductor of electricity due to its high level of electrical activities present in the lattice structure. As a result of the existence of. Although graphite is flexible, it is not elastic and has high. graphite is a fair conductor of electricity, while diamond is practically an insulator (stranger yet, it is technically classified as. Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its.
From www.numerade.com
SOLVED A piece of graphite has 10 layers each layer consisting of 40 Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its if graphite is exposed to an electric current in a circuit, the pi electrons which are already traveling between. graphite is a crystalline form of the element carbon with hexagonally arranged atoms. Each carbon atom has an. graphite is a fair conductor of electricity, while diamond is practically an insulator (stranger yet, it is technically classified as. Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its.
From www.youtube.com
Graphite Conducts Electricity YouTube Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its As a result of the existence of. graphite’s many covalent bonds are strong and substantial energy is needed to break them. Metals are generally very good conductors, meaning they let current flow easily. graphite is an excellent conductor of electricity due to its high level of electrical activities present in the lattice structure. It has free electrons that. Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its.
From askfilo.com
The graphite is a conductor electricity Filo Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its because the fourth electron of each carbon atom is unbound, graphite conducts electricity. The carbon atoms are bonded together with. graphite is an excellent conductor of electricity due to its high level of electrical activities present in the lattice structure. solids can be categorised into conductors, semiconductors or insulators by their ability to conduct electricity. Metals are. Graphite Is Either A Conductor Or An Insulator Of Electricity Depending Upon Its.