Active Electrodes Examples . Active electrodes are made of materials that actively participate in the electrochemical reaction, such as metals like platinum or gold. The electrode that actively participates in the electrochemical cell's. Active electrodes and inert electrodes. An active electrode is an electrode that is defined as the metal which is used to make electrochemical cells. Inert electrodes , like the platinum. There are two types of electrodes: Active electrodes find extensive applications in diverse areas of chemistry, including sensors, fuel cells, and electroanalytical techniques. Active electrodes can be oxidized/reduced easily in. Here we will look at some examples of electrodes. In sensor technology, active electrodes play a pivotal role in detecting and quantifying analytes by facilitating redox reactions with the target species. There are mainly two types of electrodes, namely reactive and inert electrodes.
from chem.libretexts.org
There are mainly two types of electrodes, namely reactive and inert electrodes. Active electrodes can be oxidized/reduced easily in. Active electrodes find extensive applications in diverse areas of chemistry, including sensors, fuel cells, and electroanalytical techniques. The electrode that actively participates in the electrochemical cell's. Active electrodes and inert electrodes. There are two types of electrodes: Active electrodes are made of materials that actively participate in the electrochemical reaction, such as metals like platinum or gold. An active electrode is an electrode that is defined as the metal which is used to make electrochemical cells. Here we will look at some examples of electrodes. Inert electrodes , like the platinum.
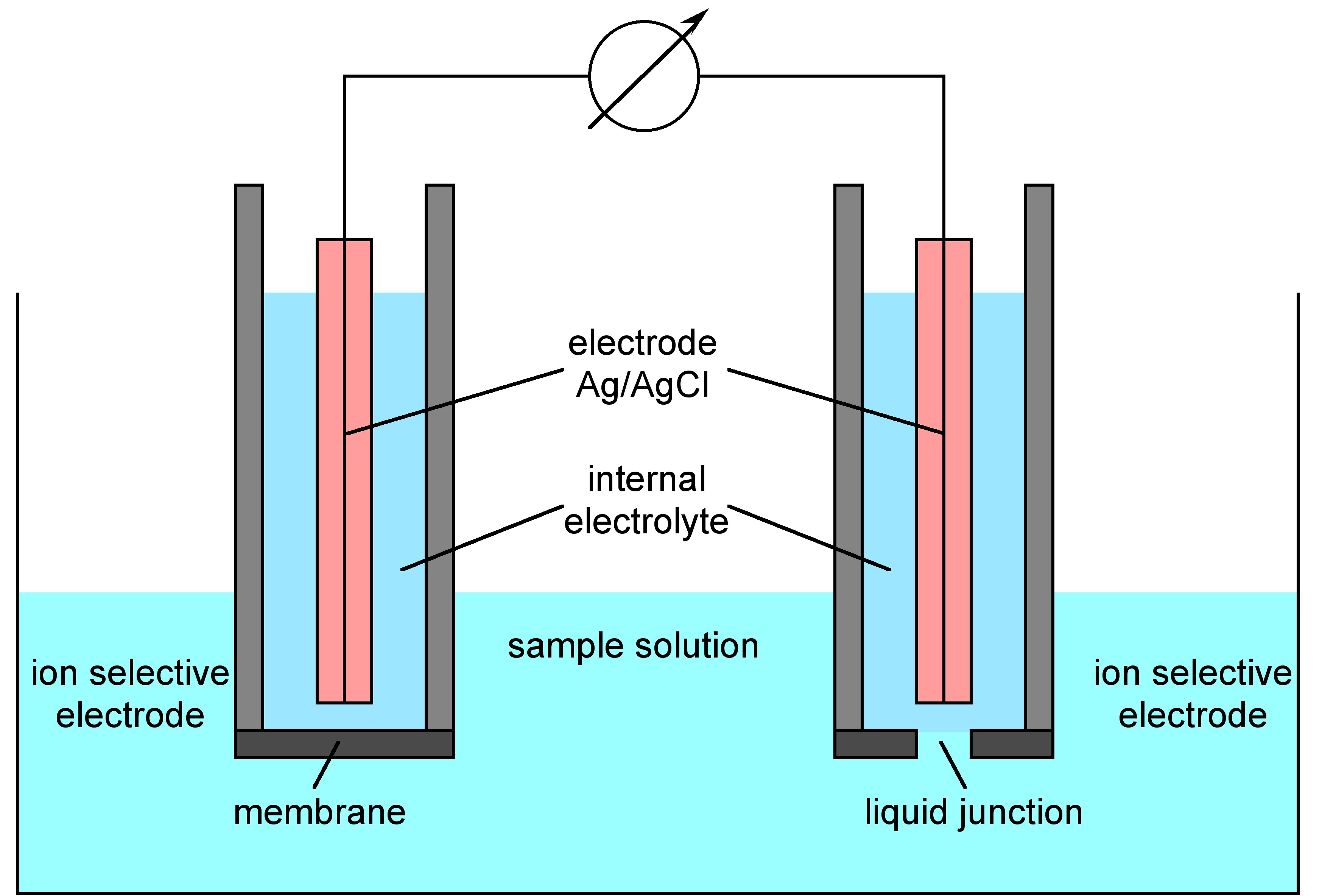
1.7 Ion Selective Electrode Analysis Chemistry LibreTexts
Active Electrodes Examples Inert electrodes , like the platinum. There are mainly two types of electrodes, namely reactive and inert electrodes. There are two types of electrodes: Here we will look at some examples of electrodes. An active electrode is an electrode that is defined as the metal which is used to make electrochemical cells. Active electrodes are made of materials that actively participate in the electrochemical reaction, such as metals like platinum or gold. Inert electrodes , like the platinum. Active electrodes can be oxidized/reduced easily in. Active electrodes find extensive applications in diverse areas of chemistry, including sensors, fuel cells, and electroanalytical techniques. Active electrodes and inert electrodes. In sensor technology, active electrodes play a pivotal role in detecting and quantifying analytes by facilitating redox reactions with the target species. The electrode that actively participates in the electrochemical cell's.
From mavink.com
Electrode Classification Diagram Active Electrodes Examples Active electrodes find extensive applications in diverse areas of chemistry, including sensors, fuel cells, and electroanalytical techniques. Active electrodes can be oxidized/reduced easily in. There are mainly two types of electrodes, namely reactive and inert electrodes. Active electrodes and inert electrodes. An active electrode is an electrode that is defined as the metal which is used to make electrochemical cells.. Active Electrodes Examples.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Active Electrodes Examples Active electrodes and inert electrodes. Active electrodes find extensive applications in diverse areas of chemistry, including sensors, fuel cells, and electroanalytical techniques. Active electrodes are made of materials that actively participate in the electrochemical reaction, such as metals like platinum or gold. There are mainly two types of electrodes, namely reactive and inert electrodes. The electrode that actively participates in. Active Electrodes Examples.
From courses.lumenlearning.com
Galvanic Cells Chemistry Atoms First Active Electrodes Examples The electrode that actively participates in the electrochemical cell's. Inert electrodes , like the platinum. Active electrodes are made of materials that actively participate in the electrochemical reaction, such as metals like platinum or gold. Here we will look at some examples of electrodes. Active electrodes find extensive applications in diverse areas of chemistry, including sensors, fuel cells, and electroanalytical. Active Electrodes Examples.
From www.researchgate.net
Schematic diagram showing thermal effect at active electrode [12 Active Electrodes Examples Active electrodes can be oxidized/reduced easily in. Here we will look at some examples of electrodes. Inert electrodes , like the platinum. Active electrodes are made of materials that actively participate in the electrochemical reaction, such as metals like platinum or gold. There are mainly two types of electrodes, namely reactive and inert electrodes. An active electrode is an electrode. Active Electrodes Examples.
From byjus.com
What is an active electrode? Explain with the help of an example Active Electrodes Examples There are mainly two types of electrodes, namely reactive and inert electrodes. There are two types of electrodes: An active electrode is an electrode that is defined as the metal which is used to make electrochemical cells. Active electrodes find extensive applications in diverse areas of chemistry, including sensors, fuel cells, and electroanalytical techniques. In sensor technology, active electrodes play. Active Electrodes Examples.
From socratic.org
Two halfcells in a galvanic cell consist of one iron (Fe(s)) electrode Active Electrodes Examples The electrode that actively participates in the electrochemical cell's. In sensor technology, active electrodes play a pivotal role in detecting and quantifying analytes by facilitating redox reactions with the target species. Inert electrodes , like the platinum. Active electrodes find extensive applications in diverse areas of chemistry, including sensors, fuel cells, and electroanalytical techniques. Active electrodes can be oxidized/reduced easily. Active Electrodes Examples.
From www.thoughtco.com
How to Define Anode and Cathode Active Electrodes Examples Active electrodes find extensive applications in diverse areas of chemistry, including sensors, fuel cells, and electroanalytical techniques. Active electrodes and inert electrodes. There are mainly two types of electrodes, namely reactive and inert electrodes. The electrode that actively participates in the electrochemical cell's. Active electrodes can be oxidized/reduced easily in. An active electrode is an electrode that is defined as. Active Electrodes Examples.
From mavink.com
Electroplating Process Flow Chart Active Electrodes Examples There are mainly two types of electrodes, namely reactive and inert electrodes. Active electrodes are made of materials that actively participate in the electrochemical reaction, such as metals like platinum or gold. The electrode that actively participates in the electrochemical cell's. In sensor technology, active electrodes play a pivotal role in detecting and quantifying analytes by facilitating redox reactions with. Active Electrodes Examples.
From www.researchgate.net
Example electrophysiological time course for two active electrodes at Active Electrodes Examples The electrode that actively participates in the electrochemical cell's. Active electrodes find extensive applications in diverse areas of chemistry, including sensors, fuel cells, and electroanalytical techniques. Active electrodes and inert electrodes. An active electrode is an electrode that is defined as the metal which is used to make electrochemical cells. Here we will look at some examples of electrodes. Inert. Active Electrodes Examples.
From chem.libretexts.org
9.4 Standard Electrode Potentials Chemistry LibreTexts Active Electrodes Examples Active electrodes find extensive applications in diverse areas of chemistry, including sensors, fuel cells, and electroanalytical techniques. An active electrode is an electrode that is defined as the metal which is used to make electrochemical cells. In sensor technology, active electrodes play a pivotal role in detecting and quantifying analytes by facilitating redox reactions with the target species. Active electrodes. Active Electrodes Examples.
From ppt-online.org
Electrolysis презентация онлайн Active Electrodes Examples There are two types of electrodes: Active electrodes can be oxidized/reduced easily in. Inert electrodes , like the platinum. Active electrodes and inert electrodes. Here we will look at some examples of electrodes. The electrode that actively participates in the electrochemical cell's. Active electrodes find extensive applications in diverse areas of chemistry, including sensors, fuel cells, and electroanalytical techniques. In. Active Electrodes Examples.
From alevelchemistry.co.uk
Electrodes Facts, Summary & Definition Chemistry Revision Active Electrodes Examples There are two types of electrodes: Inert electrodes , like the platinum. Active electrodes can be oxidized/reduced easily in. Active electrodes are made of materials that actively participate in the electrochemical reaction, such as metals like platinum or gold. There are mainly two types of electrodes, namely reactive and inert electrodes. In sensor technology, active electrodes play a pivotal role. Active Electrodes Examples.
From www.slideshare.net
Electrolysis part 3 aqueous solution Active Electrodes Examples In sensor technology, active electrodes play a pivotal role in detecting and quantifying analytes by facilitating redox reactions with the target species. Active electrodes and inert electrodes. There are two types of electrodes: The electrode that actively participates in the electrochemical cell's. Active electrodes find extensive applications in diverse areas of chemistry, including sensors, fuel cells, and electroanalytical techniques. There. Active Electrodes Examples.
From chem.libretexts.org
1.7 Ion Selective Electrode Analysis Chemistry LibreTexts Active Electrodes Examples Active electrodes can be oxidized/reduced easily in. Active electrodes are made of materials that actively participate in the electrochemical reaction, such as metals like platinum or gold. Inert electrodes , like the platinum. An active electrode is an electrode that is defined as the metal which is used to make electrochemical cells. There are two types of electrodes: Here we. Active Electrodes Examples.
From mmerevise.co.uk
Electrolysis Worksheets and Revision MME Active Electrodes Examples Active electrodes find extensive applications in diverse areas of chemistry, including sensors, fuel cells, and electroanalytical techniques. Here we will look at some examples of electrodes. The electrode that actively participates in the electrochemical cell's. An active electrode is an electrode that is defined as the metal which is used to make electrochemical cells. Active electrodes can be oxidized/reduced easily. Active Electrodes Examples.
From www.slideserve.com
PPT The ERP Boot Camp PowerPoint Presentation, free download ID1623807 Active Electrodes Examples Here we will look at some examples of electrodes. There are two types of electrodes: Active electrodes can be oxidized/reduced easily in. There are mainly two types of electrodes, namely reactive and inert electrodes. Active electrodes find extensive applications in diverse areas of chemistry, including sensors, fuel cells, and electroanalytical techniques. Inert electrodes , like the platinum. Active electrodes and. Active Electrodes Examples.
From www.slideserve.com
PPT electrolysis of solutions PowerPoint Presentation, free download Active Electrodes Examples Active electrodes are made of materials that actively participate in the electrochemical reaction, such as metals like platinum or gold. In sensor technology, active electrodes play a pivotal role in detecting and quantifying analytes by facilitating redox reactions with the target species. There are mainly two types of electrodes, namely reactive and inert electrodes. Inert electrodes , like the platinum.. Active Electrodes Examples.
From 2012books.lardbucket.org
Electrochemistry Active Electrodes Examples Here we will look at some examples of electrodes. Active electrodes and inert electrodes. In sensor technology, active electrodes play a pivotal role in detecting and quantifying analytes by facilitating redox reactions with the target species. Active electrodes find extensive applications in diverse areas of chemistry, including sensors, fuel cells, and electroanalytical techniques. An active electrode is an electrode that. Active Electrodes Examples.
From spmchemistry.blog.onlinetuition.com.my
Electrolysis of Aqueous Solution SPM Chemistry Active Electrodes Examples In sensor technology, active electrodes play a pivotal role in detecting and quantifying analytes by facilitating redox reactions with the target species. Active electrodes are made of materials that actively participate in the electrochemical reaction, such as metals like platinum or gold. Active electrodes and inert electrodes. An active electrode is an electrode that is defined as the metal which. Active Electrodes Examples.
From www.mdpi.com
J. Compos. Sci. Free FullText The Effect of Modifications of Active Electrodes Examples Active electrodes and inert electrodes. The electrode that actively participates in the electrochemical cell's. Here we will look at some examples of electrodes. Active electrodes find extensive applications in diverse areas of chemistry, including sensors, fuel cells, and electroanalytical techniques. Inert electrodes , like the platinum. There are two types of electrodes: Active electrodes are made of materials that actively. Active Electrodes Examples.
From brainly.in
There are two types of electrodes and they are Active electrode Inert Active Electrodes Examples Active electrodes are made of materials that actively participate in the electrochemical reaction, such as metals like platinum or gold. Inert electrodes , like the platinum. The electrode that actively participates in the electrochemical cell's. Active electrodes can be oxidized/reduced easily in. There are two types of electrodes: There are mainly two types of electrodes, namely reactive and inert electrodes.. Active Electrodes Examples.
From www.youtube.com
6 Different Types of Electrodes & their Reactions in Electrochemistry Active Electrodes Examples Active electrodes find extensive applications in diverse areas of chemistry, including sensors, fuel cells, and electroanalytical techniques. There are mainly two types of electrodes, namely reactive and inert electrodes. There are two types of electrodes: In sensor technology, active electrodes play a pivotal role in detecting and quantifying analytes by facilitating redox reactions with the target species. The electrode that. Active Electrodes Examples.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts Active Electrodes Examples In sensor technology, active electrodes play a pivotal role in detecting and quantifying analytes by facilitating redox reactions with the target species. Active electrodes can be oxidized/reduced easily in. Active electrodes find extensive applications in diverse areas of chemistry, including sensors, fuel cells, and electroanalytical techniques. Active electrodes and inert electrodes. Here we will look at some examples of electrodes.. Active Electrodes Examples.
From pressrelease.brainproducts.com
Brain Products' actiCAP slim active Electrodes Walkthrough Active Electrodes Examples Active electrodes are made of materials that actively participate in the electrochemical reaction, such as metals like platinum or gold. Active electrodes and inert electrodes. In sensor technology, active electrodes play a pivotal role in detecting and quantifying analytes by facilitating redox reactions with the target species. An active electrode is an electrode that is defined as the metal which. Active Electrodes Examples.
From study.com
Electrode Definition, Types & Function Lesson Active Electrodes Examples The electrode that actively participates in the electrochemical cell's. Active electrodes can be oxidized/reduced easily in. An active electrode is an electrode that is defined as the metal which is used to make electrochemical cells. There are mainly two types of electrodes, namely reactive and inert electrodes. Active electrodes are made of materials that actively participate in the electrochemical reaction,. Active Electrodes Examples.
From courses.lumenlearning.com
Electrolysis Boundless Chemistry Active Electrodes Examples Active electrodes and inert electrodes. Here we will look at some examples of electrodes. The electrode that actively participates in the electrochemical cell's. In sensor technology, active electrodes play a pivotal role in detecting and quantifying analytes by facilitating redox reactions with the target species. There are mainly two types of electrodes, namely reactive and inert electrodes. Active electrodes can. Active Electrodes Examples.
From www.researchgate.net
Examples of surface electrode types for EMG recordings (a) solid metal Active Electrodes Examples Active electrodes find extensive applications in diverse areas of chemistry, including sensors, fuel cells, and electroanalytical techniques. Inert electrodes , like the platinum. The electrode that actively participates in the electrochemical cell's. In sensor technology, active electrodes play a pivotal role in detecting and quantifying analytes by facilitating redox reactions with the target species. Active electrodes and inert electrodes. There. Active Electrodes Examples.
From www.researchgate.net
Block diagram of the 8channel activeelectrode based EEG/ETI system Active Electrodes Examples Active electrodes are made of materials that actively participate in the electrochemical reaction, such as metals like platinum or gold. Active electrodes can be oxidized/reduced easily in. In sensor technology, active electrodes play a pivotal role in detecting and quantifying analytes by facilitating redox reactions with the target species. An active electrode is an electrode that is defined as the. Active Electrodes Examples.
From www.youtube.com
Chemistry Electrolysis (Inert & Active Electrodes) YouTube Active Electrodes Examples There are two types of electrodes: An active electrode is an electrode that is defined as the metal which is used to make electrochemical cells. Active electrodes and inert electrodes. The electrode that actively participates in the electrochemical cell's. Inert electrodes , like the platinum. In sensor technology, active electrodes play a pivotal role in detecting and quantifying analytes by. Active Electrodes Examples.
From www.slideserve.com
PPT ELECTROCHEMISTRY PowerPoint Presentation, free download ID2737135 Active Electrodes Examples An active electrode is an electrode that is defined as the metal which is used to make electrochemical cells. Active electrodes find extensive applications in diverse areas of chemistry, including sensors, fuel cells, and electroanalytical techniques. There are mainly two types of electrodes, namely reactive and inert electrodes. There are two types of electrodes: Here we will look at some. Active Electrodes Examples.
From www.researchgate.net
Illustrations of a) representative examples of the active electrodes Active Electrodes Examples Active electrodes and inert electrodes. Active electrodes are made of materials that actively participate in the electrochemical reaction, such as metals like platinum or gold. In sensor technology, active electrodes play a pivotal role in detecting and quantifying analytes by facilitating redox reactions with the target species. Inert electrodes , like the platinum. Active electrodes can be oxidized/reduced easily in.. Active Electrodes Examples.
From www.differencebetween.com
Difference Between Active and Inert Electrodes Compare the Difference Active Electrodes Examples There are two types of electrodes: Active electrodes are made of materials that actively participate in the electrochemical reaction, such as metals like platinum or gold. Active electrodes find extensive applications in diverse areas of chemistry, including sensors, fuel cells, and electroanalytical techniques. Inert electrodes , like the platinum. In sensor technology, active electrodes play a pivotal role in detecting. Active Electrodes Examples.
From www.researchgate.net
Schematic illustration of a composite electrode in lithiumion Active Electrodes Examples Active electrodes and inert electrodes. Active electrodes find extensive applications in diverse areas of chemistry, including sensors, fuel cells, and electroanalytical techniques. Active electrodes can be oxidized/reduced easily in. There are two types of electrodes: In sensor technology, active electrodes play a pivotal role in detecting and quantifying analytes by facilitating redox reactions with the target species. Inert electrodes ,. Active Electrodes Examples.
From www.researchgate.net
1 Different indwelling needle electrode types. A) In the monopolar Active Electrodes Examples Active electrodes are made of materials that actively participate in the electrochemical reaction, such as metals like platinum or gold. Active electrodes find extensive applications in diverse areas of chemistry, including sensors, fuel cells, and electroanalytical techniques. In sensor technology, active electrodes play a pivotal role in detecting and quantifying analytes by facilitating redox reactions with the target species. There. Active Electrodes Examples.
From chem.libretexts.org
11.2 Standard Electrode Potentials Chemistry LibreTexts Active Electrodes Examples Active electrodes and inert electrodes. In sensor technology, active electrodes play a pivotal role in detecting and quantifying analytes by facilitating redox reactions with the target species. The electrode that actively participates in the electrochemical cell's. Active electrodes are made of materials that actively participate in the electrochemical reaction, such as metals like platinum or gold. There are two types. Active Electrodes Examples.