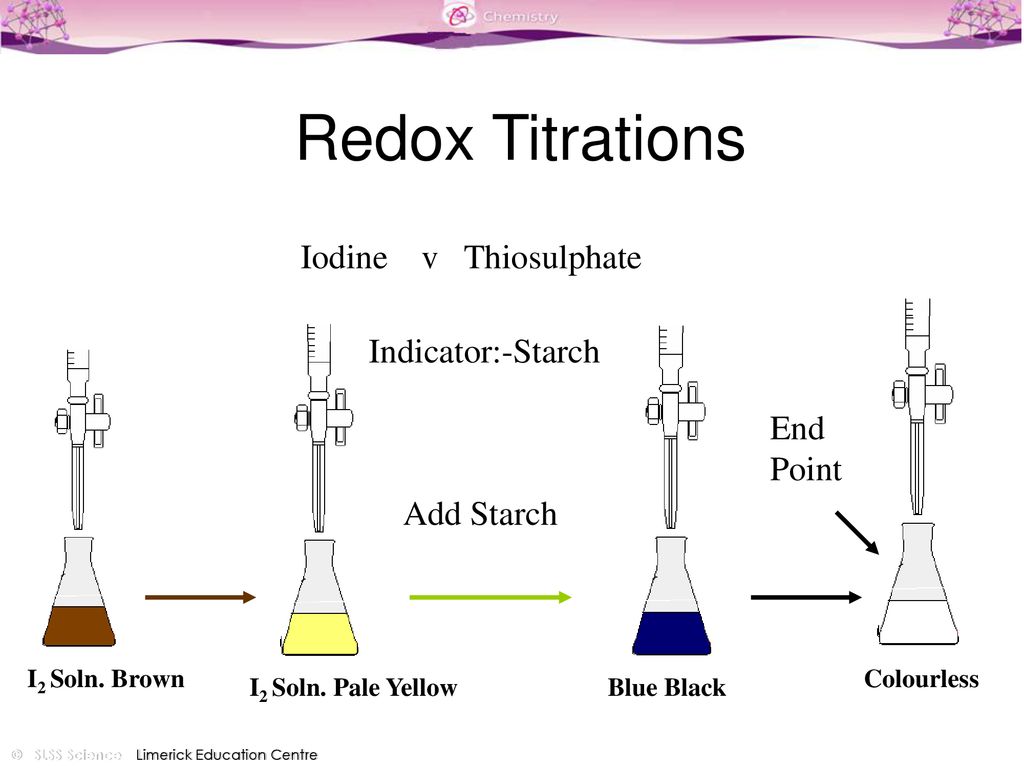
Indicator Used In Titration Of Iodine With Sodium Thiosulphate . It forms a blue/black colour with iodine. Iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a pale yellow. A redox reaction occurs between iodine and thiosulfate ions: Titrate iodine with sodium thiosulfate using starch indicator and hence estimate oxidising agents by their reaction with excess acidified potassium. As the sodium thiosulfate reacts with the iodine, the solution changes color from yellow to a lighter and more diluted shade. When the iodine has all reacted (the end point), the solution turns. Starch is used as the indicator. Redox titrations using sodium thiosulphate as a reducing agent is known as iodometric titration since it is used specifically to titrate iodine.
from slideplayer.com
Iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. A redox reaction occurs between iodine and thiosulfate ions: As the sodium thiosulfate reacts with the iodine, the solution changes color from yellow to a lighter and more diluted shade. Titrate iodine with sodium thiosulfate using starch indicator and hence estimate oxidising agents by their reaction with excess acidified potassium. Redox titrations using sodium thiosulphate as a reducing agent is known as iodometric titration since it is used specifically to titrate iodine. When the iodine has all reacted (the end point), the solution turns. Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a pale yellow. Starch is used as the indicator. It forms a blue/black colour with iodine.
Titration Colour Changes ppt download
Indicator Used In Titration Of Iodine With Sodium Thiosulphate Iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a pale yellow. Titrate iodine with sodium thiosulfate using starch indicator and hence estimate oxidising agents by their reaction with excess acidified potassium. A redox reaction occurs between iodine and thiosulfate ions: It forms a blue/black colour with iodine. As the sodium thiosulfate reacts with the iodine, the solution changes color from yellow to a lighter and more diluted shade. Starch is used as the indicator. Redox titrations using sodium thiosulphate as a reducing agent is known as iodometric titration since it is used specifically to titrate iodine. Iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. When the iodine has all reacted (the end point), the solution turns.
From slideplayer.com
Analysis of antacid By titration ppt download Indicator Used In Titration Of Iodine With Sodium Thiosulphate Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a pale yellow. It forms a blue/black colour with iodine. When the iodine has all reacted (the end point), the solution turns. A redox reaction occurs between iodine and thiosulfate ions: Starch is used as the indicator. Titrate iodine with sodium. Indicator Used In Titration Of Iodine With Sodium Thiosulphate.
From www.youtube.com
Iodine and sodium thiosulfate redox titration calculations ALevel Indicator Used In Titration Of Iodine With Sodium Thiosulphate Titrate iodine with sodium thiosulfate using starch indicator and hence estimate oxidising agents by their reaction with excess acidified potassium. Starch is used as the indicator. Redox titrations using sodium thiosulphate as a reducing agent is known as iodometric titration since it is used specifically to titrate iodine. As the sodium thiosulfate reacts with the iodine, the solution changes color. Indicator Used In Titration Of Iodine With Sodium Thiosulphate.
From www.researchgate.net
Iodine solution and sodium Thiosulphate solution. Download Table Indicator Used In Titration Of Iodine With Sodium Thiosulphate Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a pale yellow. Iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. When the iodine has all reacted (the end point), the solution turns. A redox reaction occurs between iodine and thiosulfate. Indicator Used In Titration Of Iodine With Sodium Thiosulphate.
From slideplayer.com
Volumetric Analysis OxidationReduction ppt download Indicator Used In Titration Of Iodine With Sodium Thiosulphate Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a pale yellow. A redox reaction occurs between iodine and thiosulfate ions: It forms a blue/black colour with iodine. Titrate iodine with sodium thiosulfate using starch indicator and hence estimate oxidising agents by their reaction with excess acidified potassium. Redox titrations. Indicator Used In Titration Of Iodine With Sodium Thiosulphate.
From www.youtube.com
N/10 Sodium Thiosulfate Solution Preparation and Standardization with Indicator Used In Titration Of Iodine With Sodium Thiosulphate Iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. When the iodine has all reacted (the end point), the solution turns. As the sodium thiosulfate reacts with the iodine, the solution changes color from yellow to a lighter and more diluted shade. A redox reaction occurs between iodine and thiosulfate ions:. Indicator Used In Titration Of Iodine With Sodium Thiosulphate.
From www.slideserve.com
PPT Sodium Thiosulfate Titrations PowerPoint Presentation, free Indicator Used In Titration Of Iodine With Sodium Thiosulphate When the iodine has all reacted (the end point), the solution turns. A redox reaction occurs between iodine and thiosulfate ions: Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a pale yellow. Redox titrations using sodium thiosulphate as a reducing agent is known as iodometric titration since it is. Indicator Used In Titration Of Iodine With Sodium Thiosulphate.
From www.tes.com
Iodine thiosulphate titrationA Level Chemistry for OCR A Chapter 23.3 Indicator Used In Titration Of Iodine With Sodium Thiosulphate Titrate iodine with sodium thiosulfate using starch indicator and hence estimate oxidising agents by their reaction with excess acidified potassium. Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a pale yellow. As the sodium thiosulfate reacts with the iodine, the solution changes color from yellow to a lighter and. Indicator Used In Titration Of Iodine With Sodium Thiosulphate.
From slideplayer.com
Titration Colour Changes ppt download Indicator Used In Titration Of Iodine With Sodium Thiosulphate Starch is used as the indicator. Iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. A redox reaction occurs between iodine and thiosulfate ions: It forms a blue/black colour with iodine. When the iodine has all reacted (the end point), the solution turns. Finally, we add a starch indicator to the. Indicator Used In Titration Of Iodine With Sodium Thiosulphate.
From chempedia.info
Iodine with sodium thiosulphate Big Chemical Encyclopedia Indicator Used In Titration Of Iodine With Sodium Thiosulphate A redox reaction occurs between iodine and thiosulfate ions: Iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. Starch is used as the indicator. Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a pale yellow. When the iodine has all. Indicator Used In Titration Of Iodine With Sodium Thiosulphate.
From www.youtube.com
Redox TitrationIodine solution & Sodium Thiosulphate (Na2S2O3) WAEC Indicator Used In Titration Of Iodine With Sodium Thiosulphate Redox titrations using sodium thiosulphate as a reducing agent is known as iodometric titration since it is used specifically to titrate iodine. Titrate iodine with sodium thiosulfate using starch indicator and hence estimate oxidising agents by their reaction with excess acidified potassium. It forms a blue/black colour with iodine. When the iodine has all reacted (the end point), the solution. Indicator Used In Titration Of Iodine With Sodium Thiosulphate.
From www.researchgate.net
Iodine solution and sodium Thiosulphate solution. Download Table Indicator Used In Titration Of Iodine With Sodium Thiosulphate When the iodine has all reacted (the end point), the solution turns. Redox titrations using sodium thiosulphate as a reducing agent is known as iodometric titration since it is used specifically to titrate iodine. As the sodium thiosulfate reacts with the iodine, the solution changes color from yellow to a lighter and more diluted shade. Finally, we add a starch. Indicator Used In Titration Of Iodine With Sodium Thiosulphate.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Indicator Used In Titration Of Iodine With Sodium Thiosulphate Iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. Redox titrations using sodium thiosulphate as a reducing agent is known as iodometric titration since it is used specifically to titrate iodine. Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a. Indicator Used In Titration Of Iodine With Sodium Thiosulphate.
From www.youtube.com
Iodine and sodium thiosulfate titrations YouTube Indicator Used In Titration Of Iodine With Sodium Thiosulphate When the iodine has all reacted (the end point), the solution turns. Starch is used as the indicator. Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a pale yellow. Redox titrations using sodium thiosulphate as a reducing agent is known as iodometric titration since it is used specifically to. Indicator Used In Titration Of Iodine With Sodium Thiosulphate.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Indicator Used In Titration Of Iodine With Sodium Thiosulphate It forms a blue/black colour with iodine. As the sodium thiosulfate reacts with the iodine, the solution changes color from yellow to a lighter and more diluted shade. A redox reaction occurs between iodine and thiosulfate ions: When the iodine has all reacted (the end point), the solution turns. Finally, we add a starch indicator to the solution, and the. Indicator Used In Titration Of Iodine With Sodium Thiosulphate.
From www.youtube.com
Successful titration with Thiosulfate YouTube Indicator Used In Titration Of Iodine With Sodium Thiosulphate Titrate iodine with sodium thiosulfate using starch indicator and hence estimate oxidising agents by their reaction with excess acidified potassium. Iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. It forms a blue/black colour with iodine. Starch is used as the indicator. Finally, we add a starch indicator to the solution,. Indicator Used In Titration Of Iodine With Sodium Thiosulphate.
From www.slideserve.com
PPT Chapter 7 Fat Chemistry & Analysis PowerPoint Presentation ID Indicator Used In Titration Of Iodine With Sodium Thiosulphate Iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. It forms a blue/black colour with iodine. When the iodine has all reacted (the end point), the solution turns. Redox titrations using sodium thiosulphate as a reducing agent is known as iodometric titration since it is used specifically to titrate iodine. Finally,. Indicator Used In Titration Of Iodine With Sodium Thiosulphate.
From www.slideserve.com
PPT Sodium Thiosulfate Titrations PowerPoint Presentation, free Indicator Used In Titration Of Iodine With Sodium Thiosulphate Iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. Titrate iodine with sodium thiosulfate using starch indicator and hence estimate oxidising agents by their reaction with excess acidified potassium. It forms a blue/black colour with iodine. Finally, we add a starch indicator to the solution, and the color shifts dramatically from. Indicator Used In Titration Of Iodine With Sodium Thiosulphate.
From www.youtube.com
Sodium Thiosulphate Solution preparation and Standardization Indicator Used In Titration Of Iodine With Sodium Thiosulphate Redox titrations using sodium thiosulphate as a reducing agent is known as iodometric titration since it is used specifically to titrate iodine. As the sodium thiosulfate reacts with the iodine, the solution changes color from yellow to a lighter and more diluted shade. Iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate. Indicator Used In Titration Of Iodine With Sodium Thiosulphate.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Volumetric Analysis (3) Redox Titrations Indicator Used In Titration Of Iodine With Sodium Thiosulphate A redox reaction occurs between iodine and thiosulfate ions: It forms a blue/black colour with iodine. Starch is used as the indicator. Titrate iodine with sodium thiosulfate using starch indicator and hence estimate oxidising agents by their reaction with excess acidified potassium. Redox titrations using sodium thiosulphate as a reducing agent is known as iodometric titration since it is used. Indicator Used In Titration Of Iodine With Sodium Thiosulphate.
From www.youtube.com
Iodine / Thiosulfate Redox Titration Demonstration YouTube Indicator Used In Titration Of Iodine With Sodium Thiosulphate It forms a blue/black colour with iodine. Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a pale yellow. Redox titrations using sodium thiosulphate as a reducing agent is known as iodometric titration since it is used specifically to titrate iodine. A redox reaction occurs between iodine and thiosulfate ions:. Indicator Used In Titration Of Iodine With Sodium Thiosulphate.
From fphoto.photoshelter.com
science chemistry titration iodine thiosulfate Fundamental Indicator Used In Titration Of Iodine With Sodium Thiosulphate Starch is used as the indicator. Iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. It forms a blue/black colour with iodine. Titrate iodine with sodium thiosulfate using starch indicator and hence estimate oxidising agents by their reaction with excess acidified potassium. When the iodine has all reacted (the end point),. Indicator Used In Titration Of Iodine With Sodium Thiosulphate.
From slideplayer.com
Speaking volumes Titration ppt download Indicator Used In Titration Of Iodine With Sodium Thiosulphate Iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. As the sodium thiosulfate reacts with the iodine, the solution changes color from yellow to a lighter and more diluted shade. A redox reaction occurs between iodine and thiosulfate ions: It forms a blue/black colour with iodine. Finally, we add a starch. Indicator Used In Titration Of Iodine With Sodium Thiosulphate.
From www.slideserve.com
PPT Sodium Thiosulfate Titrations PowerPoint Presentation, free Indicator Used In Titration Of Iodine With Sodium Thiosulphate Starch is used as the indicator. As the sodium thiosulfate reacts with the iodine, the solution changes color from yellow to a lighter and more diluted shade. A redox reaction occurs between iodine and thiosulfate ions: It forms a blue/black colour with iodine. Titrate iodine with sodium thiosulfate using starch indicator and hence estimate oxidising agents by their reaction with. Indicator Used In Titration Of Iodine With Sodium Thiosulphate.
From www.slideserve.com
PPT Sodium Thiosulfate Titrations PowerPoint Presentation, free Indicator Used In Titration Of Iodine With Sodium Thiosulphate Redox titrations using sodium thiosulphate as a reducing agent is known as iodometric titration since it is used specifically to titrate iodine. As the sodium thiosulfate reacts with the iodine, the solution changes color from yellow to a lighter and more diluted shade. When the iodine has all reacted (the end point), the solution turns. Titrate iodine with sodium thiosulfate. Indicator Used In Titration Of Iodine With Sodium Thiosulphate.
From www.sciencephoto.com
Iodine and thiosulfate ions titration Stock Image C033/2858 Indicator Used In Titration Of Iodine With Sodium Thiosulphate As the sodium thiosulfate reacts with the iodine, the solution changes color from yellow to a lighter and more diluted shade. A redox reaction occurs between iodine and thiosulfate ions: When the iodine has all reacted (the end point), the solution turns. Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue. Indicator Used In Titration Of Iodine With Sodium Thiosulphate.
From slideplayer.com
Volumetric Analysis OxidationReduction ppt download Indicator Used In Titration Of Iodine With Sodium Thiosulphate When the iodine has all reacted (the end point), the solution turns. Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a pale yellow. It forms a blue/black colour with iodine. As the sodium thiosulfate reacts with the iodine, the solution changes color from yellow to a lighter and more. Indicator Used In Titration Of Iodine With Sodium Thiosulphate.
From studylib.net
An iodine / thiosulfate titration Indicator Used In Titration Of Iodine With Sodium Thiosulphate Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a pale yellow. Starch is used as the indicator. Iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. As the sodium thiosulfate reacts with the iodine, the solution changes color from yellow. Indicator Used In Titration Of Iodine With Sodium Thiosulphate.
From www.studypool.com
SOLUTION Redox titrations iodine thiosulphate doc Studypool Indicator Used In Titration Of Iodine With Sodium Thiosulphate When the iodine has all reacted (the end point), the solution turns. Titrate iodine with sodium thiosulfate using starch indicator and hence estimate oxidising agents by their reaction with excess acidified potassium. A redox reaction occurs between iodine and thiosulfate ions: Starch is used as the indicator. Iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o. Indicator Used In Titration Of Iodine With Sodium Thiosulphate.
From byjus.com
Na thiosulfate in iodine titration methods Indicator Used In Titration Of Iodine With Sodium Thiosulphate Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a pale yellow. Titrate iodine with sodium thiosulfate using starch indicator and hence estimate oxidising agents by their reaction with excess acidified potassium. When the iodine has all reacted (the end point), the solution turns. A redox reaction occurs between iodine. Indicator Used In Titration Of Iodine With Sodium Thiosulphate.
From fphoto.photoshelter.com
science chemistry titration iodine thiosulfate Fundamental Indicator Used In Titration Of Iodine With Sodium Thiosulphate It forms a blue/black colour with iodine. A redox reaction occurs between iodine and thiosulfate ions: Redox titrations using sodium thiosulphate as a reducing agent is known as iodometric titration since it is used specifically to titrate iodine. Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue to a pale yellow.. Indicator Used In Titration Of Iodine With Sodium Thiosulphate.
From chem.libretexts.org
Redox Titration Chemistry LibreTexts Indicator Used In Titration Of Iodine With Sodium Thiosulphate Starch is used as the indicator. It forms a blue/black colour with iodine. Titrate iodine with sodium thiosulfate using starch indicator and hence estimate oxidising agents by their reaction with excess acidified potassium. As the sodium thiosulfate reacts with the iodine, the solution changes color from yellow to a lighter and more diluted shade. A redox reaction occurs between iodine. Indicator Used In Titration Of Iodine With Sodium Thiosulphate.
From slideplayer.com
Volumetric Analysis OxidationReduction ppt download Indicator Used In Titration Of Iodine With Sodium Thiosulphate A redox reaction occurs between iodine and thiosulfate ions: Titrate iodine with sodium thiosulfate using starch indicator and hence estimate oxidising agents by their reaction with excess acidified potassium. Redox titrations using sodium thiosulphate as a reducing agent is known as iodometric titration since it is used specifically to titrate iodine. Iodine solutions can be easily normalized against arsenic (iii). Indicator Used In Titration Of Iodine With Sodium Thiosulphate.
From www.youtube.com
Standardization of sodium thiosulphate using iodometric titration YouTube Indicator Used In Titration Of Iodine With Sodium Thiosulphate It forms a blue/black colour with iodine. Titrate iodine with sodium thiosulfate using starch indicator and hence estimate oxidising agents by their reaction with excess acidified potassium. Iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. As the sodium thiosulfate reacts with the iodine, the solution changes color from yellow to. Indicator Used In Titration Of Iodine With Sodium Thiosulphate.
From byjus.com
Oxidation state of sulphur in sodium thiosulphate during the reaction Indicator Used In Titration Of Iodine With Sodium Thiosulphate Redox titrations using sodium thiosulphate as a reducing agent is known as iodometric titration since it is used specifically to titrate iodine. Starch is used as the indicator. Titrate iodine with sodium thiosulfate using starch indicator and hence estimate oxidising agents by their reaction with excess acidified potassium. A redox reaction occurs between iodine and thiosulfate ions: It forms a. Indicator Used In Titration Of Iodine With Sodium Thiosulphate.
From www.vernier.com
Potentiometric Titration of Aqueous Iodine Indicator Used In Titration Of Iodine With Sodium Thiosulphate Titrate iodine with sodium thiosulfate using starch indicator and hence estimate oxidising agents by their reaction with excess acidified potassium. Redox titrations using sodium thiosulphate as a reducing agent is known as iodometric titration since it is used specifically to titrate iodine. Finally, we add a starch indicator to the solution, and the color shifts dramatically from a deep blue. Indicator Used In Titration Of Iodine With Sodium Thiosulphate.