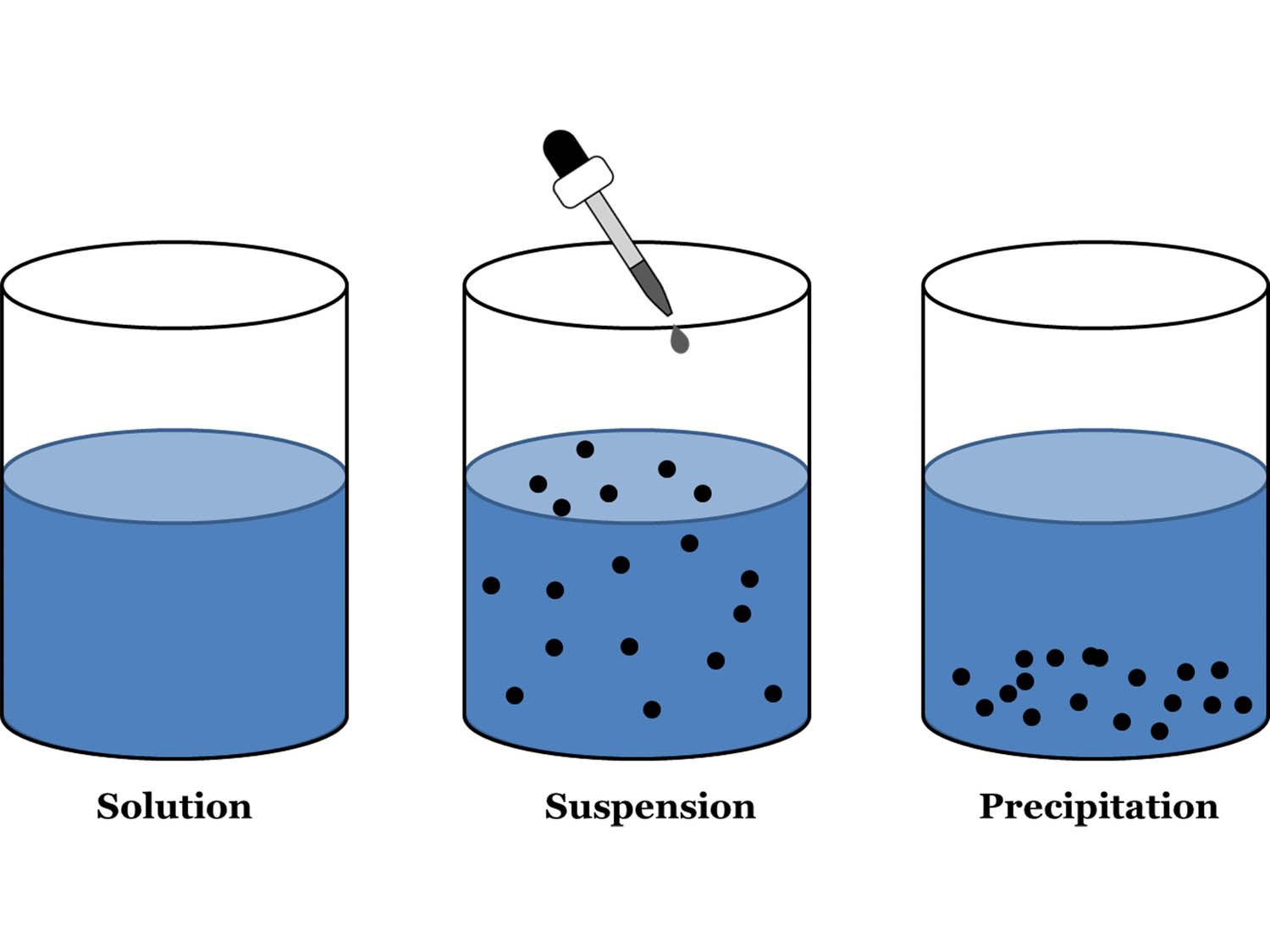
Is Salt Water A Solution Colloid Or Suspension . However, colloidal particles are small enough that they do not settle out upon. In inland waterways, clay particles, which have a charged surface, form a colloidal suspension. Salt water is neither a colloid nor a suspension. In a solution, the solute (in this case, salt) is completely dissolved in the solvent. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. The particles in a colloid are larger than most simple molecules; A good example is the way a glass of skim milk (a colloid) shows a flashlight beam, while a glass of salt water (a solution) does not. It is a quick and easy test that. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. High salt concentrations in seawater neutralize the.
from byjus.com
Salt water is neither a colloid nor a suspension. High salt concentrations in seawater neutralize the. In inland waterways, clay particles, which have a charged surface, form a colloidal suspension. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. It is a quick and easy test that. However, colloidal particles are small enough that they do not settle out upon. In a solution, the solute (in this case, salt) is completely dissolved in the solvent. A good example is the way a glass of skim milk (a colloid) shows a flashlight beam, while a glass of salt water (a solution) does not. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a.
Suspensions & Colloids Difference Between Colloid & SuspensionByju's
Is Salt Water A Solution Colloid Or Suspension It is a quick and easy test that. The particles in a colloid are larger than most simple molecules; A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. In inland waterways, clay particles, which have a charged surface, form a colloidal suspension. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. Salt water is neither a colloid nor a suspension. It is a quick and easy test that. High salt concentrations in seawater neutralize the. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. However, colloidal particles are small enough that they do not settle out upon. In a solution, the solute (in this case, salt) is completely dissolved in the solvent. A good example is the way a glass of skim milk (a colloid) shows a flashlight beam, while a glass of salt water (a solution) does not.
From www.teachoo.com
Solution Definition, Types, Properties Chemistry Teachoo Is Salt Water A Solution Colloid Or Suspension In inland waterways, clay particles, which have a charged surface, form a colloidal suspension. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. A good example is the way a glass of skim milk (a colloid) shows a flashlight beam, while a glass of salt water. Is Salt Water A Solution Colloid Or Suspension.
From www.pinterest.com
Solutions, Colloids, Suspensions Science lessons, Chemistry lessons Is Salt Water A Solution Colloid Or Suspension A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. Salt water is neither a colloid nor a suspension. However, colloidal particles are small enough that they do not settle out upon. High salt concentrations in seawater neutralize the. It is a quick and easy test that.. Is Salt Water A Solution Colloid Or Suspension.
From www.alamy.com
Solutions. homogeneous mixture. experiment with salt and water Is Salt Water A Solution Colloid Or Suspension A good example is the way a glass of skim milk (a colloid) shows a flashlight beam, while a glass of salt water (a solution) does not. However, colloidal particles are small enough that they do not settle out upon. High salt concentrations in seawater neutralize the. A solution may be colored, but it is transparent, the molecules or ions. Is Salt Water A Solution Colloid Or Suspension.
From www.majordifferences.com
Difference Between True Solutions, Colloidal solution and Suspension Is Salt Water A Solution Colloid Or Suspension The particles in a colloid are larger than most simple molecules; It is a quick and easy test that. However, colloidal particles are small enough that they do not settle out upon. In inland waterways, clay particles, which have a charged surface, form a colloidal suspension. High salt concentrations in seawater neutralize the. A colloid is a heterogeneous mixture in. Is Salt Water A Solution Colloid Or Suspension.
From www.slideserve.com
PPT 2 PowerPoint Presentation, free download ID6415741 Is Salt Water A Solution Colloid Or Suspension High salt concentrations in seawater neutralize the. In inland waterways, clay particles, which have a charged surface, form a colloidal suspension. In a solution, the solute (in this case, salt) is completely dissolved in the solvent. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Suspensions and colloids differ from. Is Salt Water A Solution Colloid Or Suspension.
From www.youtube.com
Lab Experiment on Nature of Matter Solution, Suspension and Colloid Is Salt Water A Solution Colloid Or Suspension A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. In a solution, the solute (in this case, salt) is completely dissolved in the solvent. In inland waterways, clay particles, which have a charged surface, form a colloidal suspension. A good example is the way a glass of skim milk (a. Is Salt Water A Solution Colloid Or Suspension.
From www.thoughtco.com
Tyndall Effect Definition and Examples Is Salt Water A Solution Colloid Or Suspension Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Salt water is neither a colloid nor a suspension. In a solution, the solute (in this case, salt) is completely dissolved in the. Is Salt Water A Solution Colloid Or Suspension.
From www.youtube.com
Colloids, Solutions & Suspensions YouTube Is Salt Water A Solution Colloid Or Suspension A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. The particles in a colloid are larger than most simple molecules; High salt concentrations in seawater neutralize the. In inland waterways, clay particles, which have a charged surface, form a colloidal suspension. A good example is the way a glass of. Is Salt Water A Solution Colloid Or Suspension.
From www.slideserve.com
PPT Suspensions and Colloids PowerPoint Presentation, free download Is Salt Water A Solution Colloid Or Suspension However, colloidal particles are small enough that they do not settle out upon. Salt water is neither a colloid nor a suspension. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. In inland waterways, clay particles, which have a charged surface, form a colloidal suspension. The. Is Salt Water A Solution Colloid Or Suspension.
From suspensioncolloid-00.blogspot.com
SuspensionColloid Is Salt Water A Solution Colloid Or Suspension It is a quick and easy test that. A good example is the way a glass of skim milk (a colloid) shows a flashlight beam, while a glass of salt water (a solution) does not. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. A solution may be colored, but it. Is Salt Water A Solution Colloid Or Suspension.
From www.geeksforgeeks.org
Colloids Definition, Classification, Application, Properties, & FAQs Is Salt Water A Solution Colloid Or Suspension Salt water is neither a colloid nor a suspension. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. It is a quick and easy test that. A good example is the way. Is Salt Water A Solution Colloid Or Suspension.
From www.youtube.com
SCIENCE 6 SUSPENSION AND COLLOID YouTube Is Salt Water A Solution Colloid Or Suspension In inland waterways, clay particles, which have a charged surface, form a colloidal suspension. It is a quick and easy test that. In a solution, the solute (in this case, salt) is completely dissolved in the solvent. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing.. Is Salt Water A Solution Colloid Or Suspension.
From www.youtube.com
What is solution, Suspension and Colloid? Science Composition of Is Salt Water A Solution Colloid Or Suspension However, colloidal particles are small enough that they do not settle out upon. High salt concentrations in seawater neutralize the. It is a quick and easy test that. In inland waterways, clay particles, which have a charged surface, form a colloidal suspension. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent.. Is Salt Water A Solution Colloid Or Suspension.
From sciencenotes.org
What Is a Colloid? Definition and Examples Is Salt Water A Solution Colloid Or Suspension A good example is the way a glass of skim milk (a colloid) shows a flashlight beam, while a glass of salt water (a solution) does not. It is a quick and easy test that. In a solution, the solute (in this case, salt) is completely dissolved in the solvent. A solution may be colored, but it is transparent, the. Is Salt Water A Solution Colloid Or Suspension.
From in.pinterest.com
Solutions, suspensions, and colloids Suspension mixture, Body diagram Is Salt Water A Solution Colloid Or Suspension A good example is the way a glass of skim milk (a colloid) shows a flashlight beam, while a glass of salt water (a solution) does not. High salt concentrations in seawater neutralize the. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. A solution may be colored, but it. Is Salt Water A Solution Colloid Or Suspension.
From www.geeksforgeeks.org
Colloids Definition, Properties, Classification & Examples Is Salt Water A Solution Colloid Or Suspension Salt water is neither a colloid nor a suspension. However, colloidal particles are small enough that they do not settle out upon. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size. Is Salt Water A Solution Colloid Or Suspension.
From www.myshared.ru
Презентация на тему "Colloidal systems. Classes of solution True Is Salt Water A Solution Colloid Or Suspension The particles in a colloid are larger than most simple molecules; In a solution, the solute (in this case, salt) is completely dissolved in the solvent. However, colloidal particles are small enough that they do not settle out upon. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out. Is Salt Water A Solution Colloid Or Suspension.
From chemwiki.ucdavis.edu
Figure 1 Examples of a stable and of an unstable colloidal dispersion Is Salt Water A Solution Colloid Or Suspension In a solution, the solute (in this case, salt) is completely dissolved in the solvent. In inland waterways, clay particles, which have a charged surface, form a colloidal suspension. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. Salt water is neither a colloid nor a. Is Salt Water A Solution Colloid Or Suspension.
From www.aakash.ac.in
Colloidal Solution Definition, Classification, Examples & Preparation Is Salt Water A Solution Colloid Or Suspension The particles in a colloid are larger than most simple molecules; In inland waterways, clay particles, which have a charged surface, form a colloidal suspension. High salt concentrations in seawater neutralize the. A good example is the way a glass of skim milk (a colloid) shows a flashlight beam, while a glass of salt water (a solution) does not. A. Is Salt Water A Solution Colloid Or Suspension.
From www.slideserve.com
PPT Solutions and Other Mixtures (Ch 61 and 62) PowerPoint Is Salt Water A Solution Colloid Or Suspension The particles in a colloid are larger than most simple molecules; It is a quick and easy test that. However, colloidal particles are small enough that they do not settle out upon. High salt concentrations in seawater neutralize the. In inland waterways, clay particles, which have a charged surface, form a colloidal suspension. A colloid is a heterogeneous mixture in. Is Salt Water A Solution Colloid Or Suspension.
From www.ingridscience.ca
Making mixtures solutions, suspensions and colloids ingridscience.ca Is Salt Water A Solution Colloid Or Suspension It is a quick and easy test that. In a solution, the solute (in this case, salt) is completely dissolved in the solvent. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. The particles in a colloid are larger than most simple molecules; However, colloidal particles are small enough that. Is Salt Water A Solution Colloid Or Suspension.
From slideplayer.com
Pure Substances, Mixtures, and Solutions ppt download Is Salt Water A Solution Colloid Or Suspension However, colloidal particles are small enough that they do not settle out upon. The particles in a colloid are larger than most simple molecules; High salt concentrations in seawater neutralize the. Salt water is neither a colloid nor a suspension. In a solution, the solute (in this case, salt) is completely dissolved in the solvent. A colloid is a heterogeneous. Is Salt Water A Solution Colloid Or Suspension.
From www.youtube.com
Solution Suspension Colloid YouTube Is Salt Water A Solution Colloid Or Suspension A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. The particles in a colloid are larger than most simple molecules; Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. In inland waterways, clay particles, which have a charged surface, form a. Is Salt Water A Solution Colloid Or Suspension.
From byjus.com
Suspensions & Colloids Difference Between Colloid & SuspensionByju's Is Salt Water A Solution Colloid Or Suspension A good example is the way a glass of skim milk (a colloid) shows a flashlight beam, while a glass of salt water (a solution) does not. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. In inland waterways, clay particles, which have a charged surface, form a colloidal suspension.. Is Salt Water A Solution Colloid Or Suspension.
From www.geeksforgeeks.org
Suspension(Chemistry) Definition, Properties, Examples, and FAQs Is Salt Water A Solution Colloid Or Suspension The particles in a colloid are larger than most simple molecules; A good example is the way a glass of skim milk (a colloid) shows a flashlight beam, while a glass of salt water (a solution) does not. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. A solution may be. Is Salt Water A Solution Colloid Or Suspension.
From www.slideserve.com
PPT Solution, Suspensions and Colloids PowerPoint Presentation ID Is Salt Water A Solution Colloid Or Suspension A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. It is a quick and easy test that. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. In a solution, the solute (in this case, salt) is completely. Is Salt Water A Solution Colloid Or Suspension.
From www.slideserve.com
PPT Matter Properties & Change PowerPoint Presentation, free Is Salt Water A Solution Colloid Or Suspension It is a quick and easy test that. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. Salt water is neither a colloid nor a suspension. The particles in a colloid are larger than most simple molecules; In inland waterways, clay particles, which have a charged surface, form a colloidal suspension.. Is Salt Water A Solution Colloid Or Suspension.
From www.slideshare.net
Solution suspension Is Salt Water A Solution Colloid Or Suspension In inland waterways, clay particles, which have a charged surface, form a colloidal suspension. In a solution, the solute (in this case, salt) is completely dissolved in the solvent. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. A colloid is a heterogeneous mixture in which. Is Salt Water A Solution Colloid Or Suspension.
From www.vecteezy.com
True Solution, Colloid solution and Suspension three different types of Is Salt Water A Solution Colloid Or Suspension A good example is the way a glass of skim milk (a colloid) shows a flashlight beam, while a glass of salt water (a solution) does not. High salt concentrations in seawater neutralize the. Salt water is neither a colloid nor a suspension. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those. Is Salt Water A Solution Colloid Or Suspension.
From www.slideserve.com
PPT Mixtures PowerPoint Presentation, free download ID3086482 Is Salt Water A Solution Colloid Or Suspension A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. However, colloidal particles are small enough that they do not settle out upon. A good example is the way a glass of skim milk (a colloid) shows a flashlight beam, while a glass of salt water (a. Is Salt Water A Solution Colloid Or Suspension.
From www.animalia-life.club
Examples Of Colloids Mixtures Is Salt Water A Solution Colloid Or Suspension High salt concentrations in seawater neutralize the. In inland waterways, clay particles, which have a charged surface, form a colloidal suspension. Salt water is neither a colloid nor a suspension. In a solution, the solute (in this case, salt) is completely dissolved in the solvent. A good example is the way a glass of skim milk (a colloid) shows a. Is Salt Water A Solution Colloid Or Suspension.
From www.slideserve.com
PPT CH110 Chapter 8 Solutions PowerPoint Presentation, free download Is Salt Water A Solution Colloid Or Suspension It is a quick and easy test that. Salt water is neither a colloid nor a suspension. High salt concentrations in seawater neutralize the. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. Suspensions and colloids differ from solution in that a solution is homogeneous, contains. Is Salt Water A Solution Colloid Or Suspension.
From www.slideshare.net
Solutions, suspensions, and colloids Is Salt Water A Solution Colloid Or Suspension In inland waterways, clay particles, which have a charged surface, form a colloidal suspension. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. A good example is the way a glass of skim milk (a colloid) shows a flashlight beam, while a glass of salt water. Is Salt Water A Solution Colloid Or Suspension.
From pt.slideshare.net
Solutions, suspensions, and colloids Is Salt Water A Solution Colloid Or Suspension High salt concentrations in seawater neutralize the. Suspensions and colloids differ from solution in that a solution is homogeneous, contains dissolved particles, and is translucent. A solution may be colored, but it is transparent, the molecules or ions are invisible, and they do not settle out on standing. Salt water is neither a colloid nor a suspension. In a solution,. Is Salt Water A Solution Colloid Or Suspension.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Is Salt Water A Solution Colloid Or Suspension A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. In inland waterways, clay particles, which have a charged surface, form a colloidal suspension. Salt water is neither a colloid nor a suspension. The particles in a colloid are larger than most simple molecules; It is a quick and easy test. Is Salt Water A Solution Colloid Or Suspension.