How Many Atoms Are In A Simple Cubic Unit Cell . The simple cubic unit cell may also be called “primitive cubic” and thus. This unit cell contains four atoms. The simple cubic unit cell is the simplest repeating unit in a simple cubic structure. Each corner of the unit cell is defined by a lattice point at which an atom, ion, or molecule can be found in the crystal. The cubic unit cell is the smallest repeating unit when all angles are 90 o and all lengths are equal (figure 12.1.b) with each axis. To determine this, we take the equation from the aforementioned simple cubic unit cell and add to the parenthesized six faces of the unit cell. The simplest unit cell is simple cubic (sc). Take into account that several neighbouring unit cells surround each cubic unit cell. This crystal structure is just a cube with an atom on each corner. How many nearest neighbours does each atom have?
from wisc.pb.unizin.org
The simple cubic unit cell may also be called “primitive cubic” and thus. Take into account that several neighbouring unit cells surround each cubic unit cell. The simplest unit cell is simple cubic (sc). Each corner of the unit cell is defined by a lattice point at which an atom, ion, or molecule can be found in the crystal. This unit cell contains four atoms. The cubic unit cell is the smallest repeating unit when all angles are 90 o and all lengths are equal (figure 12.1.b) with each axis. The simple cubic unit cell is the simplest repeating unit in a simple cubic structure. This crystal structure is just a cube with an atom on each corner. To determine this, we take the equation from the aforementioned simple cubic unit cell and add to the parenthesized six faces of the unit cell. How many nearest neighbours does each atom have?
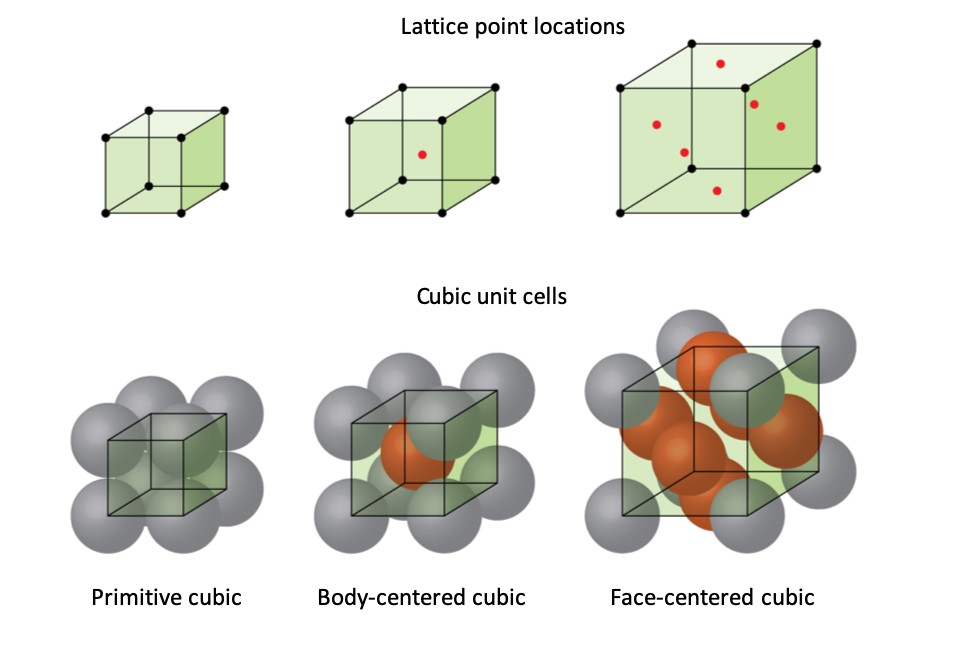
Types of Unit Cells BodyCentered Cubic and FaceCentered Cubic (M11Q5
How Many Atoms Are In A Simple Cubic Unit Cell The simple cubic unit cell is the simplest repeating unit in a simple cubic structure. The simplest unit cell is simple cubic (sc). The simple cubic unit cell is the simplest repeating unit in a simple cubic structure. This crystal structure is just a cube with an atom on each corner. Each corner of the unit cell is defined by a lattice point at which an atom, ion, or molecule can be found in the crystal. To determine this, we take the equation from the aforementioned simple cubic unit cell and add to the parenthesized six faces of the unit cell. Take into account that several neighbouring unit cells surround each cubic unit cell. How many nearest neighbours does each atom have? The simple cubic unit cell may also be called “primitive cubic” and thus. The cubic unit cell is the smallest repeating unit when all angles are 90 o and all lengths are equal (figure 12.1.b) with each axis. This unit cell contains four atoms.
From www.youtube.com
The coordination number of each atom in body centered cubic unit cell How Many Atoms Are In A Simple Cubic Unit Cell Each corner of the unit cell is defined by a lattice point at which an atom, ion, or molecule can be found in the crystal. This unit cell contains four atoms. Take into account that several neighbouring unit cells surround each cubic unit cell. The simple cubic unit cell is the simplest repeating unit in a simple cubic structure. The. How Many Atoms Are In A Simple Cubic Unit Cell.
From www.slideserve.com
PPT NUMBER OF ATOMS PER UNIT CELL IN A CUBIC LATTICE Simple cubic How Many Atoms Are In A Simple Cubic Unit Cell The simplest unit cell is simple cubic (sc). This unit cell contains four atoms. The cubic unit cell is the smallest repeating unit when all angles are 90 o and all lengths are equal (figure 12.1.b) with each axis. How many nearest neighbours does each atom have? The simple cubic unit cell is the simplest repeating unit in a simple. How Many Atoms Are In A Simple Cubic Unit Cell.
From warreninstitute.org
Exploring The Simple Cubic Unit Cell In Chemistry. How Many Atoms Are In A Simple Cubic Unit Cell This crystal structure is just a cube with an atom on each corner. The simple cubic unit cell may also be called “primitive cubic” and thus. The cubic unit cell is the smallest repeating unit when all angles are 90 o and all lengths are equal (figure 12.1.b) with each axis. Each corner of the unit cell is defined by. How Many Atoms Are In A Simple Cubic Unit Cell.
From www.slideserve.com
PPT Crystal physics PowerPoint Presentation, free download ID4024329 How Many Atoms Are In A Simple Cubic Unit Cell The cubic unit cell is the smallest repeating unit when all angles are 90 o and all lengths are equal (figure 12.1.b) with each axis. Take into account that several neighbouring unit cells surround each cubic unit cell. The simplest unit cell is simple cubic (sc). This crystal structure is just a cube with an atom on each corner. Each. How Many Atoms Are In A Simple Cubic Unit Cell.
From www.chegg.com
Solved Consider A Simple Cubic (a.k.a, Primitive Cubic) U... How Many Atoms Are In A Simple Cubic Unit Cell Take into account that several neighbouring unit cells surround each cubic unit cell. The simple cubic unit cell may also be called “primitive cubic” and thus. The cubic unit cell is the smallest repeating unit when all angles are 90 o and all lengths are equal (figure 12.1.b) with each axis. This crystal structure is just a cube with an. How Many Atoms Are In A Simple Cubic Unit Cell.
From byjus.com
what are the number of atoms per unit cell and the number of nearest How Many Atoms Are In A Simple Cubic Unit Cell The simplest unit cell is simple cubic (sc). This unit cell contains four atoms. The cubic unit cell is the smallest repeating unit when all angles are 90 o and all lengths are equal (figure 12.1.b) with each axis. Each corner of the unit cell is defined by a lattice point at which an atom, ion, or molecule can be. How Many Atoms Are In A Simple Cubic Unit Cell.
From questions.kunduz.com
Consider the simple cubic (primitive cubi... Physical Chemistry How Many Atoms Are In A Simple Cubic Unit Cell The simple cubic unit cell is the simplest repeating unit in a simple cubic structure. Take into account that several neighbouring unit cells surround each cubic unit cell. The cubic unit cell is the smallest repeating unit when all angles are 90 o and all lengths are equal (figure 12.1.b) with each axis. How many nearest neighbours does each atom. How Many Atoms Are In A Simple Cubic Unit Cell.
From www.youtube.com
Counting Atoms in a Unit Cell (BCC & FCC) YouTube How Many Atoms Are In A Simple Cubic Unit Cell Take into account that several neighbouring unit cells surround each cubic unit cell. The simple cubic unit cell may also be called “primitive cubic” and thus. This unit cell contains four atoms. The cubic unit cell is the smallest repeating unit when all angles are 90 o and all lengths are equal (figure 12.1.b) with each axis. How many nearest. How Many Atoms Are In A Simple Cubic Unit Cell.
From chem.libretexts.org
11.7 Structure of Solids Chemistry LibreTexts How Many Atoms Are In A Simple Cubic Unit Cell Each corner of the unit cell is defined by a lattice point at which an atom, ion, or molecule can be found in the crystal. This crystal structure is just a cube with an atom on each corner. The cubic unit cell is the smallest repeating unit when all angles are 90 o and all lengths are equal (figure 12.1.b). How Many Atoms Are In A Simple Cubic Unit Cell.
From www.researchgate.net
Large simple cubic unit cell with the 64 atoms listed in Table 1. The How Many Atoms Are In A Simple Cubic Unit Cell This crystal structure is just a cube with an atom on each corner. Take into account that several neighbouring unit cells surround each cubic unit cell. The cubic unit cell is the smallest repeating unit when all angles are 90 o and all lengths are equal (figure 12.1.b) with each axis. This unit cell contains four atoms. How many nearest. How Many Atoms Are In A Simple Cubic Unit Cell.
From www.researchgate.net
Unit cell of a centeredcubic structure with two atoms per cell in an How Many Atoms Are In A Simple Cubic Unit Cell The simplest unit cell is simple cubic (sc). This unit cell contains four atoms. To determine this, we take the equation from the aforementioned simple cubic unit cell and add to the parenthesized six faces of the unit cell. The simple cubic unit cell is the simplest repeating unit in a simple cubic structure. Each corner of the unit cell. How Many Atoms Are In A Simple Cubic Unit Cell.
From www.researchgate.net
4 Facecentered cubic structure (a) unit cell, (b) principal How Many Atoms Are In A Simple Cubic Unit Cell How many nearest neighbours does each atom have? Take into account that several neighbouring unit cells surround each cubic unit cell. The simple cubic unit cell may also be called “primitive cubic” and thus. Each corner of the unit cell is defined by a lattice point at which an atom, ion, or molecule can be found in the crystal. To. How Many Atoms Are In A Simple Cubic Unit Cell.
From byjus.com
In the calculation of number of atoms per unit cell, how many atoms How Many Atoms Are In A Simple Cubic Unit Cell This crystal structure is just a cube with an atom on each corner. Each corner of the unit cell is defined by a lattice point at which an atom, ion, or molecule can be found in the crystal. This unit cell contains four atoms. The cubic unit cell is the smallest repeating unit when all angles are 90 o and. How Many Atoms Are In A Simple Cubic Unit Cell.
From ar.inspiredpencil.com
Simple Cubic Unit Cell How Many Atoms Are In A Simple Cubic Unit Cell This crystal structure is just a cube with an atom on each corner. This unit cell contains four atoms. To determine this, we take the equation from the aforementioned simple cubic unit cell and add to the parenthesized six faces of the unit cell. How many nearest neighbours does each atom have? Each corner of the unit cell is defined. How Many Atoms Are In A Simple Cubic Unit Cell.
From www.chegg.com
Solved How many atoms (all are identical) are in a simple How Many Atoms Are In A Simple Cubic Unit Cell Take into account that several neighbouring unit cells surround each cubic unit cell. The simplest unit cell is simple cubic (sc). Each corner of the unit cell is defined by a lattice point at which an atom, ion, or molecule can be found in the crystal. The simple cubic unit cell is the simplest repeating unit in a simple cubic. How Many Atoms Are In A Simple Cubic Unit Cell.
From www.pinterest.com
Unit Cell Simple Cubic, Body Centered Cubic, Face Centered Cubic How Many Atoms Are In A Simple Cubic Unit Cell Each corner of the unit cell is defined by a lattice point at which an atom, ion, or molecule can be found in the crystal. The cubic unit cell is the smallest repeating unit when all angles are 90 o and all lengths are equal (figure 12.1.b) with each axis. The simple cubic unit cell may also be called “primitive. How Many Atoms Are In A Simple Cubic Unit Cell.
From www.youtube.com
HOW TO FIND NUMBER OF ATOMS IN A UNIT CELL YouTube How Many Atoms Are In A Simple Cubic Unit Cell This crystal structure is just a cube with an atom on each corner. The simple cubic unit cell may also be called “primitive cubic” and thus. Each corner of the unit cell is defined by a lattice point at which an atom, ion, or molecule can be found in the crystal. The simplest unit cell is simple cubic (sc). Take. How Many Atoms Are In A Simple Cubic Unit Cell.
From study.com
Unit Cell Lattice Parameters & Cubic Structures Video & Lesson How Many Atoms Are In A Simple Cubic Unit Cell This unit cell contains four atoms. The cubic unit cell is the smallest repeating unit when all angles are 90 o and all lengths are equal (figure 12.1.b) with each axis. This crystal structure is just a cube with an atom on each corner. To determine this, we take the equation from the aforementioned simple cubic unit cell and add. How Many Atoms Are In A Simple Cubic Unit Cell.
From www.youtube.com
What are the number of atoms per unit cell and the number of nearest How Many Atoms Are In A Simple Cubic Unit Cell Each corner of the unit cell is defined by a lattice point at which an atom, ion, or molecule can be found in the crystal. The simple cubic unit cell may also be called “primitive cubic” and thus. This crystal structure is just a cube with an atom on each corner. The cubic unit cell is the smallest repeating unit. How Many Atoms Are In A Simple Cubic Unit Cell.
From wisc.pb.unizin.org
Types of Unit Cells BodyCentered Cubic and FaceCentered Cubic (M11Q5 How Many Atoms Are In A Simple Cubic Unit Cell How many nearest neighbours does each atom have? The simplest unit cell is simple cubic (sc). This crystal structure is just a cube with an atom on each corner. This unit cell contains four atoms. Take into account that several neighbouring unit cells surround each cubic unit cell. Each corner of the unit cell is defined by a lattice point. How Many Atoms Are In A Simple Cubic Unit Cell.
From www.slideserve.com
PPT Crystalline Solids PowerPoint Presentation, free download ID How Many Atoms Are In A Simple Cubic Unit Cell The cubic unit cell is the smallest repeating unit when all angles are 90 o and all lengths are equal (figure 12.1.b) with each axis. This crystal structure is just a cube with an atom on each corner. The simplest unit cell is simple cubic (sc). The simple cubic unit cell may also be called “primitive cubic” and thus. Take. How Many Atoms Are In A Simple Cubic Unit Cell.
From www.youtube.com
Number of Atom in cubic unit cell YouTube How Many Atoms Are In A Simple Cubic Unit Cell The simple cubic unit cell may also be called “primitive cubic” and thus. How many nearest neighbours does each atom have? This unit cell contains four atoms. This crystal structure is just a cube with an atom on each corner. The cubic unit cell is the smallest repeating unit when all angles are 90 o and all lengths are equal. How Many Atoms Are In A Simple Cubic Unit Cell.
From www.doubtnut.com
How is simple cubic unit cell formed ? Calculate the number of atoms i How Many Atoms Are In A Simple Cubic Unit Cell The simple cubic unit cell may also be called “primitive cubic” and thus. This crystal structure is just a cube with an atom on each corner. Each corner of the unit cell is defined by a lattice point at which an atom, ion, or molecule can be found in the crystal. The simplest unit cell is simple cubic (sc). The. How Many Atoms Are In A Simple Cubic Unit Cell.
From kunduz.com
[ANSWERED] Consider the simple cubic primitive cubic unit cell shown in How Many Atoms Are In A Simple Cubic Unit Cell The simple cubic unit cell may also be called “primitive cubic” and thus. The cubic unit cell is the smallest repeating unit when all angles are 90 o and all lengths are equal (figure 12.1.b) with each axis. To determine this, we take the equation from the aforementioned simple cubic unit cell and add to the parenthesized six faces of. How Many Atoms Are In A Simple Cubic Unit Cell.
From saylordotorg.github.io
The Arrangement of Atoms in Crystalline Solids How Many Atoms Are In A Simple Cubic Unit Cell This unit cell contains four atoms. Take into account that several neighbouring unit cells surround each cubic unit cell. This crystal structure is just a cube with an atom on each corner. The simple cubic unit cell may also be called “primitive cubic” and thus. Each corner of the unit cell is defined by a lattice point at which an. How Many Atoms Are In A Simple Cubic Unit Cell.
From suutraceypaterson.blogspot.com
simple cubic unit cell Tracey Paterson How Many Atoms Are In A Simple Cubic Unit Cell The simplest unit cell is simple cubic (sc). To determine this, we take the equation from the aforementioned simple cubic unit cell and add to the parenthesized six faces of the unit cell. This unit cell contains four atoms. Each corner of the unit cell is defined by a lattice point at which an atom, ion, or molecule can be. How Many Atoms Are In A Simple Cubic Unit Cell.
From www.slideserve.com
PPT NUMBER OF ATOMS PER UNIT CELL IN A CUBIC LATTICE Simple cubic How Many Atoms Are In A Simple Cubic Unit Cell Take into account that several neighbouring unit cells surround each cubic unit cell. This crystal structure is just a cube with an atom on each corner. The simplest unit cell is simple cubic (sc). Each corner of the unit cell is defined by a lattice point at which an atom, ion, or molecule can be found in the crystal. This. How Many Atoms Are In A Simple Cubic Unit Cell.
From www.chegg.com
Solved Unit cells of simple cubic, body centered cubic How Many Atoms Are In A Simple Cubic Unit Cell The simplest unit cell is simple cubic (sc). The simple cubic unit cell is the simplest repeating unit in a simple cubic structure. This unit cell contains four atoms. Each corner of the unit cell is defined by a lattice point at which an atom, ion, or molecule can be found in the crystal. This crystal structure is just a. How Many Atoms Are In A Simple Cubic Unit Cell.
From www.slideserve.com
PPT CHAPTER 3 PowerPoint Presentation, free download ID472642 How Many Atoms Are In A Simple Cubic Unit Cell The cubic unit cell is the smallest repeating unit when all angles are 90 o and all lengths are equal (figure 12.1.b) with each axis. The simple cubic unit cell may also be called “primitive cubic” and thus. To determine this, we take the equation from the aforementioned simple cubic unit cell and add to the parenthesized six faces of. How Many Atoms Are In A Simple Cubic Unit Cell.
From www.youtube.com
Number of atoms/unit cell in simple cubic, fcc & bcc Solid State How Many Atoms Are In A Simple Cubic Unit Cell The cubic unit cell is the smallest repeating unit when all angles are 90 o and all lengths are equal (figure 12.1.b) with each axis. The simplest unit cell is simple cubic (sc). The simple cubic unit cell is the simplest repeating unit in a simple cubic structure. The simple cubic unit cell may also be called “primitive cubic” and. How Many Atoms Are In A Simple Cubic Unit Cell.
From byjus.com
how to find contribution by an atom in cubic unit cell How Many Atoms Are In A Simple Cubic Unit Cell The cubic unit cell is the smallest repeating unit when all angles are 90 o and all lengths are equal (figure 12.1.b) with each axis. Take into account that several neighbouring unit cells surround each cubic unit cell. To determine this, we take the equation from the aforementioned simple cubic unit cell and add to the parenthesized six faces of. How Many Atoms Are In A Simple Cubic Unit Cell.
From www.youtube.com
Unit Cell and Crystal Structure SIMPLE CUBIC , BODY CENTER CUBIC, FACE How Many Atoms Are In A Simple Cubic Unit Cell This unit cell contains four atoms. The simplest unit cell is simple cubic (sc). To determine this, we take the equation from the aforementioned simple cubic unit cell and add to the parenthesized six faces of the unit cell. Take into account that several neighbouring unit cells surround each cubic unit cell. Each corner of the unit cell is defined. How Many Atoms Are In A Simple Cubic Unit Cell.
From www.chegg.com
Solved Consider A Bodycentered Cubic Unit Cell As Shown How Many Atoms Are In A Simple Cubic Unit Cell The simple cubic unit cell is the simplest repeating unit in a simple cubic structure. The simplest unit cell is simple cubic (sc). The cubic unit cell is the smallest repeating unit when all angles are 90 o and all lengths are equal (figure 12.1.b) with each axis. To determine this, we take the equation from the aforementioned simple cubic. How Many Atoms Are In A Simple Cubic Unit Cell.
From www.youtube.com
Unit Cell Chemistry Membership YouTube How Many Atoms Are In A Simple Cubic Unit Cell The simple cubic unit cell may also be called “primitive cubic” and thus. This unit cell contains four atoms. The cubic unit cell is the smallest repeating unit when all angles are 90 o and all lengths are equal (figure 12.1.b) with each axis. Each corner of the unit cell is defined by a lattice point at which an atom,. How Many Atoms Are In A Simple Cubic Unit Cell.
From www.youtube.com
3cubic unit cell & number of atoms in a cubic unit cell, simple cubic How Many Atoms Are In A Simple Cubic Unit Cell The simple cubic unit cell may also be called “primitive cubic” and thus. The cubic unit cell is the smallest repeating unit when all angles are 90 o and all lengths are equal (figure 12.1.b) with each axis. The simple cubic unit cell is the simplest repeating unit in a simple cubic structure. Each corner of the unit cell is. How Many Atoms Are In A Simple Cubic Unit Cell.