Fda Drug Interaction Studies . The new 2020 clinical ddi guidance from the fda now has even higher harmonization with the guidance (or guidelines) from the ema and pmda. The recent fda draft drug interaction guidance highlighted the in vitro models and criteria that may be used to guide further in vivo drug. This guideline provides recommendation to promote a consistent approach in designing, conducting, and interpreting enzyme. The guidance is intended to harmonize the regional recommendations for designing, conducting, and interpreting in vitro and clinical. Index substrates listed in this table were selected considering their sensitivity, specificity, safety profiles, and adequate number.
from mavink.com
The new 2020 clinical ddi guidance from the fda now has even higher harmonization with the guidance (or guidelines) from the ema and pmda. This guideline provides recommendation to promote a consistent approach in designing, conducting, and interpreting enzyme. Index substrates listed in this table were selected considering their sensitivity, specificity, safety profiles, and adequate number. The recent fda draft drug interaction guidance highlighted the in vitro models and criteria that may be used to guide further in vivo drug. The guidance is intended to harmonize the regional recommendations for designing, conducting, and interpreting in vitro and clinical.
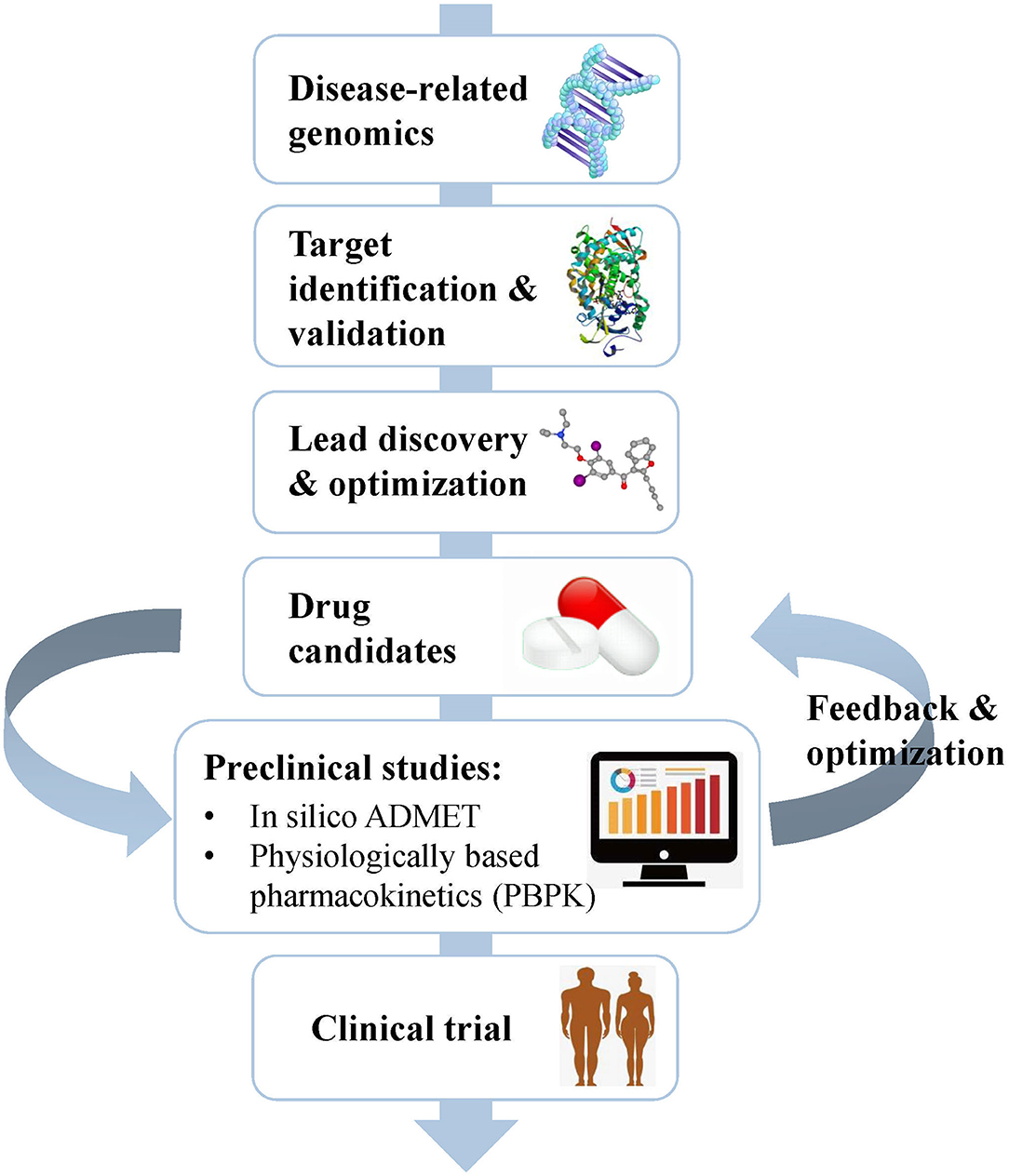
Drug Discovery Flowchart
Fda Drug Interaction Studies The guidance is intended to harmonize the regional recommendations for designing, conducting, and interpreting in vitro and clinical. The new 2020 clinical ddi guidance from the fda now has even higher harmonization with the guidance (or guidelines) from the ema and pmda. Index substrates listed in this table were selected considering their sensitivity, specificity, safety profiles, and adequate number. The recent fda draft drug interaction guidance highlighted the in vitro models and criteria that may be used to guide further in vivo drug. This guideline provides recommendation to promote a consistent approach in designing, conducting, and interpreting enzyme. The guidance is intended to harmonize the regional recommendations for designing, conducting, and interpreting in vitro and clinical.
From prorelixresearch.com
IND Data Requirements and US FDA Submission Process Fda Drug Interaction Studies Index substrates listed in this table were selected considering their sensitivity, specificity, safety profiles, and adequate number. This guideline provides recommendation to promote a consistent approach in designing, conducting, and interpreting enzyme. The guidance is intended to harmonize the regional recommendations for designing, conducting, and interpreting in vitro and clinical. The new 2020 clinical ddi guidance from the fda now. Fda Drug Interaction Studies.
From www.finnegan.com
FDA Draft Guidance on DrugDrug Interaction Assessment for Therapeutic Fda Drug Interaction Studies Index substrates listed in this table were selected considering their sensitivity, specificity, safety profiles, and adequate number. The guidance is intended to harmonize the regional recommendations for designing, conducting, and interpreting in vitro and clinical. The new 2020 clinical ddi guidance from the fda now has even higher harmonization with the guidance (or guidelines) from the ema and pmda. The. Fda Drug Interaction Studies.
From blog.biobide.com
FDA Drug Approval Process How Drugs are Developed and Approved Fda Drug Interaction Studies The guidance is intended to harmonize the regional recommendations for designing, conducting, and interpreting in vitro and clinical. The recent fda draft drug interaction guidance highlighted the in vitro models and criteria that may be used to guide further in vivo drug. The new 2020 clinical ddi guidance from the fda now has even higher harmonization with the guidance (or. Fda Drug Interaction Studies.
From www.certara.com
Deciding on Which Drugdrug Interactions to Evaluate in the Clinic Fda Drug Interaction Studies Index substrates listed in this table were selected considering their sensitivity, specificity, safety profiles, and adequate number. The guidance is intended to harmonize the regional recommendations for designing, conducting, and interpreting in vitro and clinical. The recent fda draft drug interaction guidance highlighted the in vitro models and criteria that may be used to guide further in vivo drug. This. Fda Drug Interaction Studies.
From www.goodrx.com
The 10 Warfarin Interactions to Be Aware Of GoodRx Fda Drug Interaction Studies The guidance is intended to harmonize the regional recommendations for designing, conducting, and interpreting in vitro and clinical. This guideline provides recommendation to promote a consistent approach in designing, conducting, and interpreting enzyme. The recent fda draft drug interaction guidance highlighted the in vitro models and criteria that may be used to guide further in vivo drug. The new 2020. Fda Drug Interaction Studies.
From www.researchgate.net
Drugdrug interaction of baricitinib. Download Scientific Diagram Fda Drug Interaction Studies The new 2020 clinical ddi guidance from the fda now has even higher harmonization with the guidance (or guidelines) from the ema and pmda. The guidance is intended to harmonize the regional recommendations for designing, conducting, and interpreting in vitro and clinical. The recent fda draft drug interaction guidance highlighted the in vitro models and criteria that may be used. Fda Drug Interaction Studies.
From sponsors.drvince.com
Overview of DrugDrug Interaction Studies at Dr. Vince Clinical Fda Drug Interaction Studies Index substrates listed in this table were selected considering their sensitivity, specificity, safety profiles, and adequate number. This guideline provides recommendation to promote a consistent approach in designing, conducting, and interpreting enzyme. The recent fda draft drug interaction guidance highlighted the in vitro models and criteria that may be used to guide further in vivo drug. The new 2020 clinical. Fda Drug Interaction Studies.
From www.hematologyadvisor.com
FDA Drug and Biological Approvals 2023 Recap Hematology Advisor Fda Drug Interaction Studies Index substrates listed in this table were selected considering their sensitivity, specificity, safety profiles, and adequate number. The recent fda draft drug interaction guidance highlighted the in vitro models and criteria that may be used to guide further in vivo drug. The new 2020 clinical ddi guidance from the fda now has even higher harmonization with the guidance (or guidelines). Fda Drug Interaction Studies.
From www.xenotech.com
2020 FDA Final Guidance, "In Vitro Drug Interaction Studies Fda Drug Interaction Studies The recent fda draft drug interaction guidance highlighted the in vitro models and criteria that may be used to guide further in vivo drug. This guideline provides recommendation to promote a consistent approach in designing, conducting, and interpreting enzyme. The new 2020 clinical ddi guidance from the fda now has even higher harmonization with the guidance (or guidelines) from the. Fda Drug Interaction Studies.
From www.researchgate.net
(PDF) Drug Interaction Fda Drug Interaction Studies The new 2020 clinical ddi guidance from the fda now has even higher harmonization with the guidance (or guidelines) from the ema and pmda. This guideline provides recommendation to promote a consistent approach in designing, conducting, and interpreting enzyme. The recent fda draft drug interaction guidance highlighted the in vitro models and criteria that may be used to guide further. Fda Drug Interaction Studies.
From deal.town
Clinical Pharmacology Corner FDA’s SBIA Announces inars on Drug Fda Drug Interaction Studies Index substrates listed in this table were selected considering their sensitivity, specificity, safety profiles, and adequate number. The guidance is intended to harmonize the regional recommendations for designing, conducting, and interpreting in vitro and clinical. This guideline provides recommendation to promote a consistent approach in designing, conducting, and interpreting enzyme. The new 2020 clinical ddi guidance from the fda now. Fda Drug Interaction Studies.
From www.frontiersin.org
Frontiers The role of drug transporters in the kidney lessons from Fda Drug Interaction Studies Index substrates listed in this table were selected considering their sensitivity, specificity, safety profiles, and adequate number. The guidance is intended to harmonize the regional recommendations for designing, conducting, and interpreting in vitro and clinical. This guideline provides recommendation to promote a consistent approach in designing, conducting, and interpreting enzyme. The new 2020 clinical ddi guidance from the fda now. Fda Drug Interaction Studies.
From www.semanticscholar.org
FOODDRUG INTERACTION AND THEIR CLINICAL IMPLICATIONS SELECTED Fda Drug Interaction Studies The new 2020 clinical ddi guidance from the fda now has even higher harmonization with the guidance (or guidelines) from the ema and pmda. This guideline provides recommendation to promote a consistent approach in designing, conducting, and interpreting enzyme. Index substrates listed in this table were selected considering their sensitivity, specificity, safety profiles, and adequate number. The recent fda draft. Fda Drug Interaction Studies.
From www.researchgate.net
Drugdrug interaction network evaluation with clopidogrel as Fda Drug Interaction Studies The recent fda draft drug interaction guidance highlighted the in vitro models and criteria that may be used to guide further in vivo drug. The guidance is intended to harmonize the regional recommendations for designing, conducting, and interpreting in vitro and clinical. The new 2020 clinical ddi guidance from the fda now has even higher harmonization with the guidance (or. Fda Drug Interaction Studies.
From academicentrepreneurship.pubpub.org
FDA Drug Regulation Investigational New Drug Applications · Academic Fda Drug Interaction Studies The new 2020 clinical ddi guidance from the fda now has even higher harmonization with the guidance (or guidelines) from the ema and pmda. The recent fda draft drug interaction guidance highlighted the in vitro models and criteria that may be used to guide further in vivo drug. Index substrates listed in this table were selected considering their sensitivity, specificity,. Fda Drug Interaction Studies.
From www.smartsheet.com
How to Create Approval Processes Smartsheet Fda Drug Interaction Studies The new 2020 clinical ddi guidance from the fda now has even higher harmonization with the guidance (or guidelines) from the ema and pmda. This guideline provides recommendation to promote a consistent approach in designing, conducting, and interpreting enzyme. Index substrates listed in this table were selected considering their sensitivity, specificity, safety profiles, and adequate number. The guidance is intended. Fda Drug Interaction Studies.
From www.semanticscholar.org
[PDF] Guidance for Industry Drug Interaction Studies — Study Design Fda Drug Interaction Studies Index substrates listed in this table were selected considering their sensitivity, specificity, safety profiles, and adequate number. The recent fda draft drug interaction guidance highlighted the in vitro models and criteria that may be used to guide further in vivo drug. This guideline provides recommendation to promote a consistent approach in designing, conducting, and interpreting enzyme. The guidance is intended. Fda Drug Interaction Studies.
From www.researchgate.net
OF METHODS FOR DRUGDRUG INTERACTION DETECTION Download Scientific Fda Drug Interaction Studies The recent fda draft drug interaction guidance highlighted the in vitro models and criteria that may be used to guide further in vivo drug. The new 2020 clinical ddi guidance from the fda now has even higher harmonization with the guidance (or guidelines) from the ema and pmda. Index substrates listed in this table were selected considering their sensitivity, specificity,. Fda Drug Interaction Studies.
From www.imperialcrs.com
Clinical Research Phases and the Path to Drug Approval Imperial Fda Drug Interaction Studies This guideline provides recommendation to promote a consistent approach in designing, conducting, and interpreting enzyme. The recent fda draft drug interaction guidance highlighted the in vitro models and criteria that may be used to guide further in vivo drug. The new 2020 clinical ddi guidance from the fda now has even higher harmonization with the guidance (or guidelines) from the. Fda Drug Interaction Studies.
From ddblog.labcorp.com
DrugDrug Interaction (DDI) 2020 FDA Guidance A Quick Summary Fda Drug Interaction Studies Index substrates listed in this table were selected considering their sensitivity, specificity, safety profiles, and adequate number. This guideline provides recommendation to promote a consistent approach in designing, conducting, and interpreting enzyme. The new 2020 clinical ddi guidance from the fda now has even higher harmonization with the guidance (or guidelines) from the ema and pmda. The guidance is intended. Fda Drug Interaction Studies.
From www.cc.nih.gov
FDA Responses and Meetings for Investigational New Drug Applications Fda Drug Interaction Studies The new 2020 clinical ddi guidance from the fda now has even higher harmonization with the guidance (or guidelines) from the ema and pmda. The recent fda draft drug interaction guidance highlighted the in vitro models and criteria that may be used to guide further in vivo drug. Index substrates listed in this table were selected considering their sensitivity, specificity,. Fda Drug Interaction Studies.
From www.linkedin.com
FDA Drug Topics Biosimilars LinkedIn Fda Drug Interaction Studies The new 2020 clinical ddi guidance from the fda now has even higher harmonization with the guidance (or guidelines) from the ema and pmda. The guidance is intended to harmonize the regional recommendations for designing, conducting, and interpreting in vitro and clinical. The recent fda draft drug interaction guidance highlighted the in vitro models and criteria that may be used. Fda Drug Interaction Studies.
From learn.issx.org
International Society for the Study of Xenobiotics Update on FDA's Fda Drug Interaction Studies The new 2020 clinical ddi guidance from the fda now has even higher harmonization with the guidance (or guidelines) from the ema and pmda. Index substrates listed in this table were selected considering their sensitivity, specificity, safety profiles, and adequate number. This guideline provides recommendation to promote a consistent approach in designing, conducting, and interpreting enzyme. The recent fda draft. Fda Drug Interaction Studies.
From www.qcaremed.com
FDA Drug Approval Dr. David R Buyer, MD, MBA, FACP Fda Drug Interaction Studies Index substrates listed in this table were selected considering their sensitivity, specificity, safety profiles, and adequate number. The new 2020 clinical ddi guidance from the fda now has even higher harmonization with the guidance (or guidelines) from the ema and pmda. This guideline provides recommendation to promote a consistent approach in designing, conducting, and interpreting enzyme. The guidance is intended. Fda Drug Interaction Studies.
From www.researchgate.net
Drugdrug interaction (DDI) Prediction Pipeline Overview. (Step 1 Fda Drug Interaction Studies This guideline provides recommendation to promote a consistent approach in designing, conducting, and interpreting enzyme. The guidance is intended to harmonize the regional recommendations for designing, conducting, and interpreting in vitro and clinical. The recent fda draft drug interaction guidance highlighted the in vitro models and criteria that may be used to guide further in vivo drug. The new 2020. Fda Drug Interaction Studies.
From www.pharma-iq.com
FDA Draft Guidance 2012 Drug Interaction Studies Study Design, Data Fda Drug Interaction Studies The recent fda draft drug interaction guidance highlighted the in vitro models and criteria that may be used to guide further in vivo drug. Index substrates listed in this table were selected considering their sensitivity, specificity, safety profiles, and adequate number. The guidance is intended to harmonize the regional recommendations for designing, conducting, and interpreting in vitro and clinical. This. Fda Drug Interaction Studies.
From www.pragmatyxs.com
How the FDA handles drug shortages Pragmatyxs Fda Drug Interaction Studies The recent fda draft drug interaction guidance highlighted the in vitro models and criteria that may be used to guide further in vivo drug. The guidance is intended to harmonize the regional recommendations for designing, conducting, and interpreting in vitro and clinical. The new 2020 clinical ddi guidance from the fda now has even higher harmonization with the guidance (or. Fda Drug Interaction Studies.
From www.koreabiomed.com
‘FDA’s formula for predicting interactions between drugs inaccurate’ Fda Drug Interaction Studies Index substrates listed in this table were selected considering their sensitivity, specificity, safety profiles, and adequate number. The new 2020 clinical ddi guidance from the fda now has even higher harmonization with the guidance (or guidelines) from the ema and pmda. The guidance is intended to harmonize the regional recommendations for designing, conducting, and interpreting in vitro and clinical. This. Fda Drug Interaction Studies.
From www.slideserve.com
PPT Bayes PK Models and Applications to Drug Interaction Simulations Fda Drug Interaction Studies The recent fda draft drug interaction guidance highlighted the in vitro models and criteria that may be used to guide further in vivo drug. The new 2020 clinical ddi guidance from the fda now has even higher harmonization with the guidance (or guidelines) from the ema and pmda. This guideline provides recommendation to promote a consistent approach in designing, conducting,. Fda Drug Interaction Studies.
From www.biocurate.com
The U.S. Food and Drug Administration Guidance Snapshots Biocurate Fda Drug Interaction Studies The guidance is intended to harmonize the regional recommendations for designing, conducting, and interpreting in vitro and clinical. This guideline provides recommendation to promote a consistent approach in designing, conducting, and interpreting enzyme. Index substrates listed in this table were selected considering their sensitivity, specificity, safety profiles, and adequate number. The new 2020 clinical ddi guidance from the fda now. Fda Drug Interaction Studies.
From deneyimbelgesi.blogspot.com
Fda drug products Fda Drug Interaction Studies The recent fda draft drug interaction guidance highlighted the in vitro models and criteria that may be used to guide further in vivo drug. The new 2020 clinical ddi guidance from the fda now has even higher harmonization with the guidance (or guidelines) from the ema and pmda. This guideline provides recommendation to promote a consistent approach in designing, conducting,. Fda Drug Interaction Studies.
From mavink.com
Drug Discovery Flowchart Fda Drug Interaction Studies Index substrates listed in this table were selected considering their sensitivity, specificity, safety profiles, and adequate number. The guidance is intended to harmonize the regional recommendations for designing, conducting, and interpreting in vitro and clinical. The recent fda draft drug interaction guidance highlighted the in vitro models and criteria that may be used to guide further in vivo drug. This. Fda Drug Interaction Studies.
From www.slideserve.com
PPT Drugdrug Interaction Studies PowerPoint Presentation, free Fda Drug Interaction Studies The guidance is intended to harmonize the regional recommendations for designing, conducting, and interpreting in vitro and clinical. Index substrates listed in this table were selected considering their sensitivity, specificity, safety profiles, and adequate number. This guideline provides recommendation to promote a consistent approach in designing, conducting, and interpreting enzyme. The recent fda draft drug interaction guidance highlighted the in. Fda Drug Interaction Studies.
From www.researchgate.net
(PDF) Clinical Studies on Drug‐Drug Interactions Involving Metabolism Fda Drug Interaction Studies Index substrates listed in this table were selected considering their sensitivity, specificity, safety profiles, and adequate number. The guidance is intended to harmonize the regional recommendations for designing, conducting, and interpreting in vitro and clinical. The recent fda draft drug interaction guidance highlighted the in vitro models and criteria that may be used to guide further in vivo drug. The. Fda Drug Interaction Studies.
From lifesciences.csoftintl.com
FDA Provides New Guidance for DrugDrug Interaction Studies of Fda Drug Interaction Studies Index substrates listed in this table were selected considering their sensitivity, specificity, safety profiles, and adequate number. The guidance is intended to harmonize the regional recommendations for designing, conducting, and interpreting in vitro and clinical. The new 2020 clinical ddi guidance from the fda now has even higher harmonization with the guidance (or guidelines) from the ema and pmda. The. Fda Drug Interaction Studies.