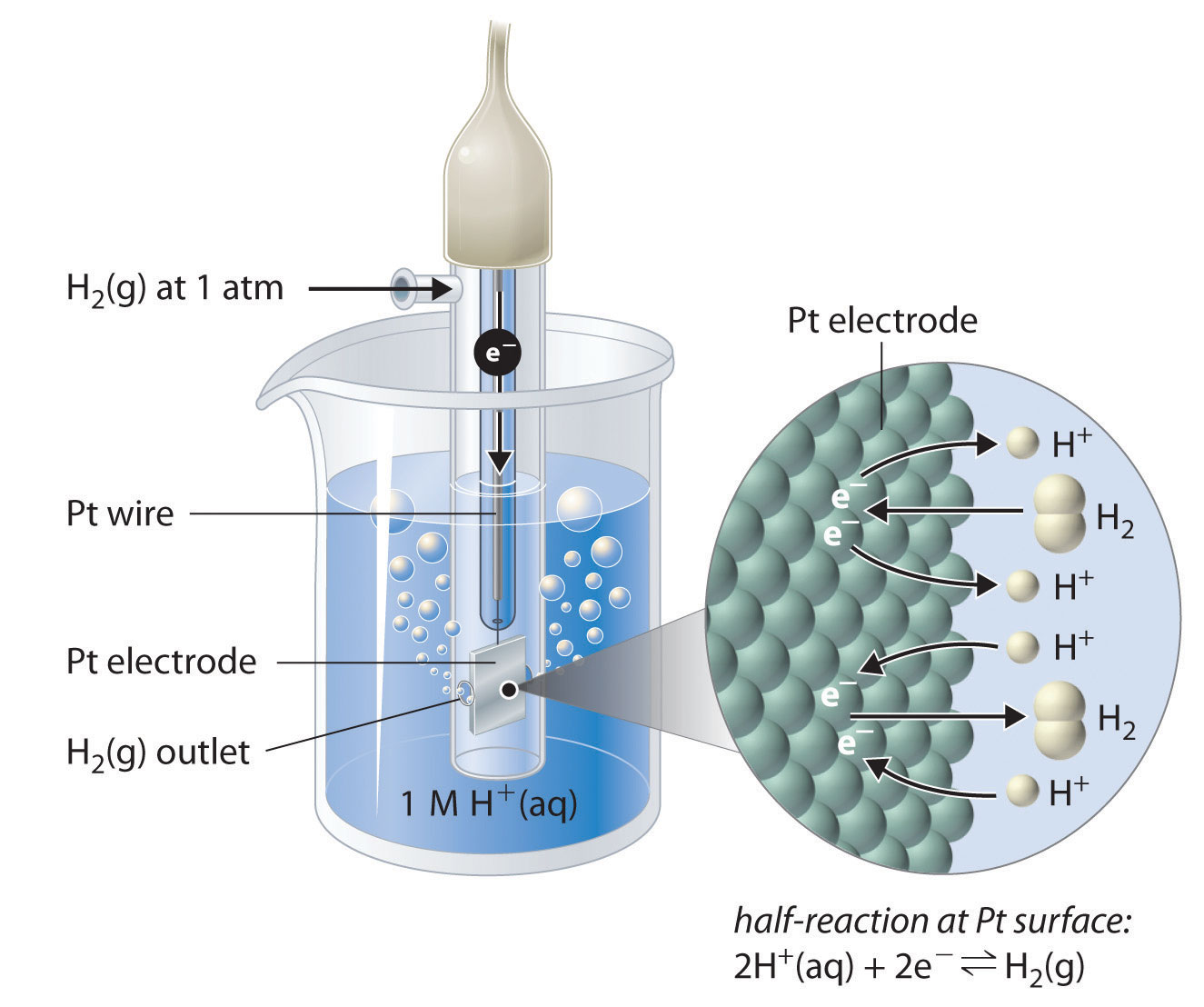
Why Standard Hydrogen Electrode Is Used . Standard hydrogen electrode is used as a primary reference electrode to know the standard electrode potentials (relative) of. The value of the standard electrode potential is. Could any other element be used instead? Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. Why is the hydrogen electrode used as the reference to set electrode potentials? The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of Examples of using the standard hydrogen electrode to determine The structure of the standard hydrogen electrode is described. What is a standard hydrogen electrode?
from saylordotorg.github.io
What is a standard hydrogen electrode? The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. The value of the standard electrode potential is. Standard hydrogen electrode is used as a primary reference electrode to know the standard electrode potentials (relative) of. Examples of using the standard hydrogen electrode to determine Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. Why is the hydrogen electrode used as the reference to set electrode potentials? Could any other element be used instead? The structure of the standard hydrogen electrode is described.
Electrochemistry
Why Standard Hydrogen Electrode Is Used Examples of using the standard hydrogen electrode to determine The value of the standard electrode potential is. The structure of the standard hydrogen electrode is described. Standard hydrogen electrode is used as a primary reference electrode to know the standard electrode potentials (relative) of. What is a standard hydrogen electrode? Why is the hydrogen electrode used as the reference to set electrode potentials? Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. Examples of using the standard hydrogen electrode to determine Could any other element be used instead?
From gaskatel.de
Reference electrode Gaskatel Why Standard Hydrogen Electrode Is Used The value of the standard electrode potential is. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. The structure of the standard hydrogen electrode is described. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at. Why Standard Hydrogen Electrode Is Used.
From www.youtube.com
Define standard hydrogen electrode SHE and write the reactions that Why Standard Hydrogen Electrode Is Used Could any other element be used instead? Why is the hydrogen electrode used as the reference to set electrode potentials? Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of The. Why Standard Hydrogen Electrode Is Used.
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE Why Standard Hydrogen Electrode Is Used The structure of the standard hydrogen electrode is described. The value of the standard electrode potential is. Examples of using the standard hydrogen electrode to determine Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. Could any other element be used instead? Standard hydrogen electrode is used as a primary. Why Standard Hydrogen Electrode Is Used.
From www.shutterstock.com
Diagramme standard d'électrode à hydrogène. Illustration image Why Standard Hydrogen Electrode Is Used What is a standard hydrogen electrode? Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of Could any other element be used instead? Standard hydrogen electrode is used as a reference. Why Standard Hydrogen Electrode Is Used.
From joixdjzhs.blob.core.windows.net
Hydrogen Electrode Process at Byron Banks blog Why Standard Hydrogen Electrode Is Used The value of the standard electrode potential is. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of Could any other element be used instead? The structure of the standard hydrogen. Why Standard Hydrogen Electrode Is Used.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID752723 Why Standard Hydrogen Electrode Is Used Standard hydrogen electrode is used as a primary reference electrode to know the standard electrode potentials (relative) of. Why is the hydrogen electrode used as the reference to set electrode potentials? Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. What is a standard hydrogen electrode? The structure of the. Why Standard Hydrogen Electrode Is Used.
From classnotes.org.in
Electrode Potential and E.M.F. of a Galvanic Cell Chemistry, Class 12 Why Standard Hydrogen Electrode Is Used The structure of the standard hydrogen electrode is described. Examples of using the standard hydrogen electrode to determine Standard hydrogen electrode is used as a primary reference electrode to know the standard electrode potentials (relative) of. Could any other element be used instead? Why is the hydrogen electrode used as the reference to set electrode potentials? Although the standard hydrogen. Why Standard Hydrogen Electrode Is Used.
From loegddcbq.blob.core.windows.net
Standard Hydrogen Electrode In English at Janice Wagner blog Why Standard Hydrogen Electrode Is Used Examples of using the standard hydrogen electrode to determine Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of The value of the standard electrode potential is. Could any other element. Why Standard Hydrogen Electrode Is Used.
From www.youtube.com
Standard Hydrogen Electrode (SHE)/ Primary reference electrode Gas Why Standard Hydrogen Electrode Is Used Examples of using the standard hydrogen electrode to determine Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. Standard hydrogen electrode is used as a primary reference electrode to know the standard electrode potentials (relative) of. The value of the standard electrode potential is. Although the standard hydrogen electrode is. Why Standard Hydrogen Electrode Is Used.
From exofvrmmu.blob.core.windows.net
Standard Hydrogen Electrode Problems at Samuel Moton blog Why Standard Hydrogen Electrode Is Used Why is the hydrogen electrode used as the reference to set electrode potentials? Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. Standard hydrogen electrode is used as a primary reference electrode to know the standard electrode potentials (relative) of. What is a standard hydrogen electrode? The value of the. Why Standard Hydrogen Electrode Is Used.
From gaskatel.de
Principles of the pHvalue measurement with hydrogen electrodes Gaskatel Why Standard Hydrogen Electrode Is Used Examples of using the standard hydrogen electrode to determine The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. What is a standard hydrogen electrode? Could any other element be used instead? Although the standard hydrogen electrode is one of the most fundamental concepts of physical. Why Standard Hydrogen Electrode Is Used.
From brainly.in
what is standard hydrogen electrode draw Brainly.in Why Standard Hydrogen Electrode Is Used Why is the hydrogen electrode used as the reference to set electrode potentials? The structure of the standard hydrogen electrode is described. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. Standard hydrogen electrode is used as a primary reference electrode to know the standard electrode potentials (relative) of. What. Why Standard Hydrogen Electrode Is Used.
From scienceinfo.com
What is Standard Hydrogen Electrode? Why Standard Hydrogen Electrode Is Used The value of the standard electrode potential is. What is a standard hydrogen electrode? Why is the hydrogen electrode used as the reference to set electrode potentials? Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. Could any other element be used instead? Although the standard hydrogen electrode is one. Why Standard Hydrogen Electrode Is Used.
From byjus.com
What is standard hydrogen electrode? Why Standard Hydrogen Electrode Is Used What is a standard hydrogen electrode? Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. The structure of the standard hydrogen electrode is described. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. The value. Why Standard Hydrogen Electrode Is Used.
From www.youtube.com
Standard Hydrogen Electrode Demonstration YouTube Why Standard Hydrogen Electrode Is Used The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. The value of the standard electrode potential is. Why is the hydrogen electrode used as the reference. Why Standard Hydrogen Electrode Is Used.
From stock.adobe.com
A Standard Hydrogen Electrode (SHE) is an electrode that scientists use Why Standard Hydrogen Electrode Is Used What is a standard hydrogen electrode? The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Examples of using the standard hydrogen electrode to determine The structure of the standard hydrogen electrode is described. Standard hydrogen electrode is used as a primary reference electrode to know. Why Standard Hydrogen Electrode Is Used.
From loegddcbq.blob.core.windows.net
Standard Hydrogen Electrode In English at Janice Wagner blog Why Standard Hydrogen Electrode Is Used The structure of the standard hydrogen electrode is described. Could any other element be used instead? Why is the hydrogen electrode used as the reference to set electrode potentials? Examples of using the standard hydrogen electrode to determine Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. The standard hydrogen. Why Standard Hydrogen Electrode Is Used.
From dxojwpdmy.blob.core.windows.net
Hydrogen Electrode Explanation at Valerie Hewes blog Why Standard Hydrogen Electrode Is Used What is a standard hydrogen electrode? The structure of the standard hydrogen electrode is described. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of Examples of using the standard hydrogen. Why Standard Hydrogen Electrode Is Used.
From www.chegg.com
Solved The standard hydrogen electrode (SHE) consists of a Why Standard Hydrogen Electrode Is Used Standard hydrogen electrode is used as a primary reference electrode to know the standard electrode potentials (relative) of. What is a standard hydrogen electrode? Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and. Why Standard Hydrogen Electrode Is Used.
From www.linstitute.net
IB DP Chemistry HL复习笔记19.1.2 The Standard Hydrogen Electrode翰林国际教育 Why Standard Hydrogen Electrode Is Used Examples of using the standard hydrogen electrode to determine Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of Why is the hydrogen electrode used as the reference to set electrode. Why Standard Hydrogen Electrode Is Used.
From brainly.in
working of standard hydrogen electrode Brainly.in Why Standard Hydrogen Electrode Is Used Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. The value of the standard electrode potential is. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the. Why Standard Hydrogen Electrode Is Used.
From joihprueb.blob.core.windows.net
Hydrogen Electrode Physical Chemistry at Clifford Bart blog Why Standard Hydrogen Electrode Is Used The structure of the standard hydrogen electrode is described. Could any other element be used instead? Why is the hydrogen electrode used as the reference to set electrode potentials? Examples of using the standard hydrogen electrode to determine The value of the standard electrode potential is. Although the standard hydrogen electrode is one of the most fundamental concepts of physical. Why Standard Hydrogen Electrode Is Used.
From saylordotorg.github.io
Electrochemistry Why Standard Hydrogen Electrode Is Used Examples of using the standard hydrogen electrode to determine What is a standard hydrogen electrode? Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. Could any other element be used instead? The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0. Why Standard Hydrogen Electrode Is Used.
From dxojwpdmy.blob.core.windows.net
Hydrogen Electrode Explanation at Valerie Hewes blog Why Standard Hydrogen Electrode Is Used What is a standard hydrogen electrode? The value of the standard electrode potential is. Could any other element be used instead? The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential. Why Standard Hydrogen Electrode Is Used.
From brainly.in
What is standard hydrogen electrode? How is it prepared? Explain with Why Standard Hydrogen Electrode Is Used Examples of using the standard hydrogen electrode to determine What is a standard hydrogen electrode? Standard hydrogen electrode is used as a primary reference electrode to know the standard electrode potentials (relative) of. The value of the standard electrode potential is. The structure of the standard hydrogen electrode is described. Why is the hydrogen electrode used as the reference to. Why Standard Hydrogen Electrode Is Used.
From byjus.com
Standard Hydrogen Electrode Definition, Construction, and Labelled Why Standard Hydrogen Electrode Is Used Standard hydrogen electrode is used as a primary reference electrode to know the standard electrode potentials (relative) of. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. What is a standard hydrogen electrode? The value of the standard electrode potential is. Standard hydrogen electrode is. Why Standard Hydrogen Electrode Is Used.
From mydigitalkemistry.com
Why Standard Hydrogen Electrode act both as a Cathode and Anode Why Standard Hydrogen Electrode Is Used Standard hydrogen electrode is used as a primary reference electrode to know the standard electrode potentials (relative) of. Could any other element be used instead? The structure of the standard hydrogen electrode is described. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Examples of. Why Standard Hydrogen Electrode Is Used.
From chemistnotes.com
Standard hydrogen electrode(SHE) Definition, diagram, application Why Standard Hydrogen Electrode Is Used Could any other element be used instead? Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated. Why Standard Hydrogen Electrode Is Used.
From www.vecteezy.com
A Standard Hydrogen Electrode SHE is an electrode that scientists use Why Standard Hydrogen Electrode Is Used What is a standard hydrogen electrode? Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is. Why Standard Hydrogen Electrode Is Used.
From www.youtube.com
19.1 Standard hydrogen electrode (HL) YouTube Why Standard Hydrogen Electrode Is Used The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. The value of the standard electrode potential is. The structure of the standard hydrogen electrode is described. Examples of using the standard hydrogen electrode to determine Why is the hydrogen electrode used as the reference to. Why Standard Hydrogen Electrode Is Used.
From www.numerade.com
SOLVED Explain Standard Hydrogen Electrode (SHE) or Normal Hydrogen Why Standard Hydrogen Electrode Is Used Could any other element be used instead? The value of the standard electrode potential is. Why is the hydrogen electrode used as the reference to set electrode potentials? Standard hydrogen electrode is used as a primary reference electrode to know the standard electrode potentials (relative) of. The structure of the standard hydrogen electrode is described. Examples of using the standard. Why Standard Hydrogen Electrode Is Used.
From dxojwpdmy.blob.core.windows.net
Hydrogen Electrode Explanation at Valerie Hewes blog Why Standard Hydrogen Electrode Is Used What is a standard hydrogen electrode? Why is the hydrogen electrode used as the reference to set electrode potentials? The structure of the standard hydrogen electrode is described. Examples of using the standard hydrogen electrode to determine Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. The value of the. Why Standard Hydrogen Electrode Is Used.
From socratic.org
Two halfcells in a galvanic cell consist of one iron (Fe(s)) electrode Why Standard Hydrogen Electrode Is Used Why is the hydrogen electrode used as the reference to set electrode potentials? Examples of using the standard hydrogen electrode to determine The structure of the standard hydrogen electrode is described. The value of the standard electrode potential is. What is a standard hydrogen electrode? Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential. Why Standard Hydrogen Electrode Is Used.
From twitter.com
Digital Kemistry Free Online Chemistry Learning on Twitter "Do you Why Standard Hydrogen Electrode Is Used What is a standard hydrogen electrode? The structure of the standard hydrogen electrode is described. Why is the hydrogen electrode used as the reference to set electrode potentials? Examples of using the standard hydrogen electrode to determine Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. Standard hydrogen electrode is. Why Standard Hydrogen Electrode Is Used.
From saylordotorg.github.io
Electrochemistry Why Standard Hydrogen Electrode Is Used Standard hydrogen electrode is used as a primary reference electrode to know the standard electrode potentials (relative) of. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of Examples of using. Why Standard Hydrogen Electrode Is Used.