Binding Energy Vs Relative Number Of Electrons . you will find the difference is equal to the electron’s mass to three digits, implying the binding energy is small in comparison. the definition of the binding energy of an atom to a solid is the difference between the total energy of the solid without the atom. the relative stability of a nucleus is correlated with its binding energy per nucleon, the total binding energy for the. 7 rows the atomic binding energy derives from the electromagnetic interaction of the electrons with the nucleus, mediated by. the binding energy is the amount of energy required to eject a particular electron transforming it from a bound state in a specific orbital. the binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. Compare the binding energy of a nucleon in a nucleus to the ionization energy of an electron in an atom. calculate the mass defect and binding energy for a wide range of nuclei. the photoelectron spectrum is a plot of the relative number of electrons emitted versus their ionization energy. Use a graph of binding energy per nucleon (ben) versus mass number (a) graph to assess the relative stability of a nucleus.
from alevelchemistry.co.uk
the photoelectron spectrum is a plot of the relative number of electrons emitted versus their ionization energy. the definition of the binding energy of an atom to a solid is the difference between the total energy of the solid without the atom. the binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. Compare the binding energy of a nucleon in a nucleus to the ionization energy of an electron in an atom. the binding energy is the amount of energy required to eject a particular electron transforming it from a bound state in a specific orbital. the relative stability of a nucleus is correlated with its binding energy per nucleon, the total binding energy for the. calculate the mass defect and binding energy for a wide range of nuclei. you will find the difference is equal to the electron’s mass to three digits, implying the binding energy is small in comparison. 7 rows the atomic binding energy derives from the electromagnetic interaction of the electrons with the nucleus, mediated by. Use a graph of binding energy per nucleon (ben) versus mass number (a) graph to assess the relative stability of a nucleus.
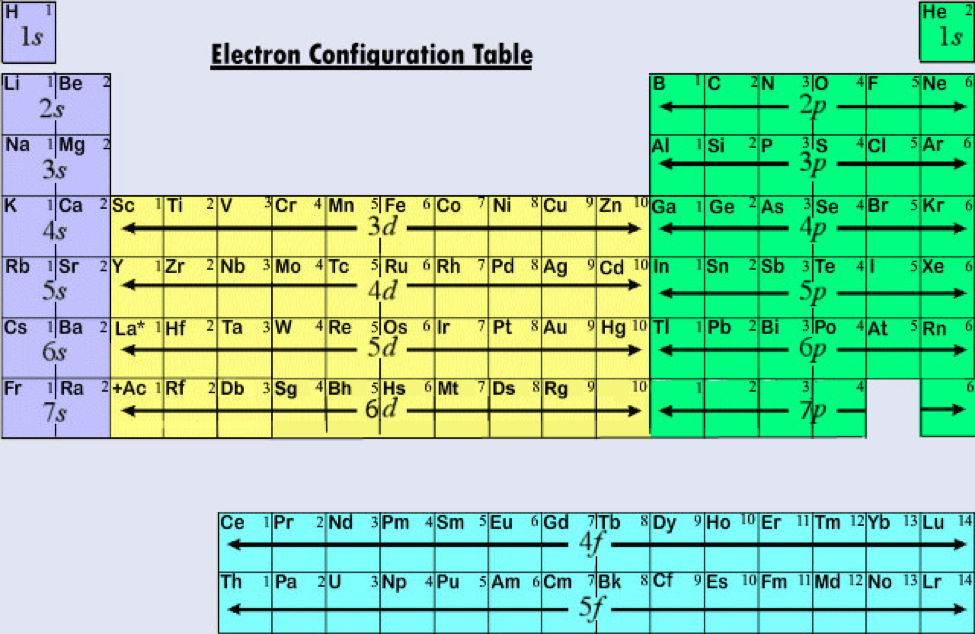
Electron Configurations Orbitals, Energy Levels and Ionisation Energy
Binding Energy Vs Relative Number Of Electrons 7 rows the atomic binding energy derives from the electromagnetic interaction of the electrons with the nucleus, mediated by. the photoelectron spectrum is a plot of the relative number of electrons emitted versus their ionization energy. the definition of the binding energy of an atom to a solid is the difference between the total energy of the solid without the atom. Compare the binding energy of a nucleon in a nucleus to the ionization energy of an electron in an atom. the binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. the binding energy is the amount of energy required to eject a particular electron transforming it from a bound state in a specific orbital. you will find the difference is equal to the electron’s mass to three digits, implying the binding energy is small in comparison. the relative stability of a nucleus is correlated with its binding energy per nucleon, the total binding energy for the. 7 rows the atomic binding energy derives from the electromagnetic interaction of the electrons with the nucleus, mediated by. calculate the mass defect and binding energy for a wide range of nuclei. Use a graph of binding energy per nucleon (ben) versus mass number (a) graph to assess the relative stability of a nucleus.
From www.doubtnut.com
Draw a plot showing the variation of binding energy per nucleon versus Binding Energy Vs Relative Number Of Electrons the binding energy is the amount of energy required to eject a particular electron transforming it from a bound state in a specific orbital. 7 rows the atomic binding energy derives from the electromagnetic interaction of the electrons with the nucleus, mediated by. the binding energy (be) of a nucleus is the energy needed to separate it. Binding Energy Vs Relative Number Of Electrons.
From curiophysics.com
Binding Energy Per Nucleon Binding Energy Curve » Curio Physics Binding Energy Vs Relative Number Of Electrons Compare the binding energy of a nucleon in a nucleus to the ionization energy of an electron in an atom. the relative stability of a nucleus is correlated with its binding energy per nucleon, the total binding energy for the. the binding energy is the amount of energy required to eject a particular electron transforming it from a. Binding Energy Vs Relative Number Of Electrons.
From www.researchgate.net
Binding energy versus number of HCN molecules for the stepwise Binding Energy Vs Relative Number Of Electrons Use a graph of binding energy per nucleon (ben) versus mass number (a) graph to assess the relative stability of a nucleus. the binding energy is the amount of energy required to eject a particular electron transforming it from a bound state in a specific orbital. the relative stability of a nucleus is correlated with its binding energy. Binding Energy Vs Relative Number Of Electrons.
From alevelchemistry.co.uk
Electron Configurations Orbitals, Energy Levels and Ionisation Energy Binding Energy Vs Relative Number Of Electrons Use a graph of binding energy per nucleon (ben) versus mass number (a) graph to assess the relative stability of a nucleus. the photoelectron spectrum is a plot of the relative number of electrons emitted versus their ionization energy. calculate the mass defect and binding energy for a wide range of nuclei. Compare the binding energy of a. Binding Energy Vs Relative Number Of Electrons.
From www.slideserve.com
PPT Chapter 21 PowerPoint Presentation, free download ID5479794 Binding Energy Vs Relative Number Of Electrons Compare the binding energy of a nucleon in a nucleus to the ionization energy of an electron in an atom. the binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. the binding energy is the amount of energy required to eject a particular electron transforming it from a bound. Binding Energy Vs Relative Number Of Electrons.
From www.researchgate.net
Molecular orbitals, electron binding energy and energy for Binding Energy Vs Relative Number Of Electrons calculate the mass defect and binding energy for a wide range of nuclei. 7 rows the atomic binding energy derives from the electromagnetic interaction of the electrons with the nucleus, mediated by. Compare the binding energy of a nucleon in a nucleus to the ionization energy of an electron in an atom. the definition of the binding. Binding Energy Vs Relative Number Of Electrons.
From www.researchgate.net
(a) Shows the intensity of photoelectrons versus binding energy from a Binding Energy Vs Relative Number Of Electrons the definition of the binding energy of an atom to a solid is the difference between the total energy of the solid without the atom. calculate the mass defect and binding energy for a wide range of nuclei. Compare the binding energy of a nucleon in a nucleus to the ionization energy of an electron in an atom.. Binding Energy Vs Relative Number Of Electrons.
From stock.adobe.com
nuclear binding energy curve, Graph of Binding Energy per Nucleon vs Binding Energy Vs Relative Number Of Electrons the definition of the binding energy of an atom to a solid is the difference between the total energy of the solid without the atom. 7 rows the atomic binding energy derives from the electromagnetic interaction of the electrons with the nucleus, mediated by. the photoelectron spectrum is a plot of the relative number of electrons emitted. Binding Energy Vs Relative Number Of Electrons.
From www.universetoday.com
What is Binding Energy? Universe Today Binding Energy Vs Relative Number Of Electrons the binding energy is the amount of energy required to eject a particular electron transforming it from a bound state in a specific orbital. the relative stability of a nucleus is correlated with its binding energy per nucleon, the total binding energy for the. the definition of the binding energy of an atom to a solid is. Binding Energy Vs Relative Number Of Electrons.
From www.slideserve.com
PPT Atomic and Nuclear Physics (7) PowerPoint Presentation, free Binding Energy Vs Relative Number Of Electrons the photoelectron spectrum is a plot of the relative number of electrons emitted versus their ionization energy. the binding energy is the amount of energy required to eject a particular electron transforming it from a bound state in a specific orbital. the definition of the binding energy of an atom to a solid is the difference between. Binding Energy Vs Relative Number Of Electrons.
From phys.libretexts.org
12.3 Nuclear Binding Energy Physics LibreTexts Binding Energy Vs Relative Number Of Electrons the definition of the binding energy of an atom to a solid is the difference between the total energy of the solid without the atom. 7 rows the atomic binding energy derives from the electromagnetic interaction of the electrons with the nucleus, mediated by. you will find the difference is equal to the electron’s mass to three. Binding Energy Vs Relative Number Of Electrons.
From www.researchgate.net
4. Binding Energy per Nucleon vs. Mass Number. Download Scientific Binding Energy Vs Relative Number Of Electrons the relative stability of a nucleus is correlated with its binding energy per nucleon, the total binding energy for the. 7 rows the atomic binding energy derives from the electromagnetic interaction of the electrons with the nucleus, mediated by. the photoelectron spectrum is a plot of the relative number of electrons emitted versus their ionization energy. . Binding Energy Vs Relative Number Of Electrons.
From www.slideserve.com
PPT Part 2 PowerPoint Presentation, free download ID298452 Binding Energy Vs Relative Number Of Electrons calculate the mass defect and binding energy for a wide range of nuclei. 7 rows the atomic binding energy derives from the electromagnetic interaction of the electrons with the nucleus, mediated by. Compare the binding energy of a nucleon in a nucleus to the ionization energy of an electron in an atom. the photoelectron spectrum is a. Binding Energy Vs Relative Number Of Electrons.
From www.slideserve.com
PPT Physics of Radiography Chapter 4 PowerPoint Presentation, free Binding Energy Vs Relative Number Of Electrons the binding energy is the amount of energy required to eject a particular electron transforming it from a bound state in a specific orbital. the relative stability of a nucleus is correlated with its binding energy per nucleon, the total binding energy for the. the definition of the binding energy of an atom to a solid is. Binding Energy Vs Relative Number Of Electrons.
From courses.lumenlearning.com
Nuclear Structure and Stability Chemistry for Majors Binding Energy Vs Relative Number Of Electrons the binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. you will find the difference is equal to the electron’s mass to three digits, implying the binding energy is small in comparison. the photoelectron spectrum is a plot of the relative number of electrons emitted versus their ionization. Binding Energy Vs Relative Number Of Electrons.
From www.chegg.com
Solved Relative Number of Electrons 0 6 5 4 3 2 1 Binding Binding Energy Vs Relative Number Of Electrons the binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. you will find the difference is equal to the electron’s mass to three digits, implying the binding energy is small in comparison. Use a graph of binding energy per nucleon (ben) versus mass number (a) graph to assess the. Binding Energy Vs Relative Number Of Electrons.
From www.britannica.com
Nuclear binding energy Definition, Formula, Mass Defect, & Graph Binding Energy Vs Relative Number Of Electrons calculate the mass defect and binding energy for a wide range of nuclei. Use a graph of binding energy per nucleon (ben) versus mass number (a) graph to assess the relative stability of a nucleus. 7 rows the atomic binding energy derives from the electromagnetic interaction of the electrons with the nucleus, mediated by. the binding energy. Binding Energy Vs Relative Number Of Electrons.
From pressbooks.online.ucf.edu
31.6 Binding Energy College Physics Binding Energy Vs Relative Number Of Electrons the binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. Compare the binding energy of a nucleon in a nucleus to the ionization energy of an electron in an atom. Use a graph of binding energy per nucleon (ben) versus mass number (a) graph to assess the relative stability of. Binding Energy Vs Relative Number Of Electrons.
From www.globalsino.com
Binding energy Practical Electron Microscopy and Database An Online Binding Energy Vs Relative Number Of Electrons the relative stability of a nucleus is correlated with its binding energy per nucleon, the total binding energy for the. the binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. calculate the mass defect and binding energy for a wide range of nuclei. the photoelectron spectrum is. Binding Energy Vs Relative Number Of Electrons.
From www.researchgate.net
Binding energy as a function of the nucleus mass. Download Binding Energy Vs Relative Number Of Electrons the photoelectron spectrum is a plot of the relative number of electrons emitted versus their ionization energy. you will find the difference is equal to the electron’s mass to three digits, implying the binding energy is small in comparison. the binding energy is the amount of energy required to eject a particular electron transforming it from a. Binding Energy Vs Relative Number Of Electrons.
From general.chemistrysteps.com
Nuclear Binding Energy Chemistry Steps Binding Energy Vs Relative Number Of Electrons the photoelectron spectrum is a plot of the relative number of electrons emitted versus their ionization energy. the binding energy is the amount of energy required to eject a particular electron transforming it from a bound state in a specific orbital. Use a graph of binding energy per nucleon (ben) versus mass number (a) graph to assess the. Binding Energy Vs Relative Number Of Electrons.
From www.sliderbase.com
Energy Levels, Sublevels, Electrons Binding Energy Vs Relative Number Of Electrons the relative stability of a nucleus is correlated with its binding energy per nucleon, the total binding energy for the. the binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. Use a graph of binding energy per nucleon (ben) versus mass number (a) graph to assess the relative stability. Binding Energy Vs Relative Number Of Electrons.
From www.chegg.com
Solved Relative Number of Electrons 100 Binding Energy Binding Energy Vs Relative Number Of Electrons the definition of the binding energy of an atom to a solid is the difference between the total energy of the solid without the atom. the relative stability of a nucleus is correlated with its binding energy per nucleon, the total binding energy for the. the binding energy is the amount of energy required to eject a. Binding Energy Vs Relative Number Of Electrons.
From www.wizeprep.com
Nuclear Forces and Binding Energy Wize University Physics Textbook Binding Energy Vs Relative Number Of Electrons the photoelectron spectrum is a plot of the relative number of electrons emitted versus their ionization energy. Use a graph of binding energy per nucleon (ben) versus mass number (a) graph to assess the relative stability of a nucleus. Compare the binding energy of a nucleon in a nucleus to the ionization energy of an electron in an atom.. Binding Energy Vs Relative Number Of Electrons.
From questions-in.kunduz.com
2. Binding energy per nucleon vs mass number curve f... Physics Binding Energy Vs Relative Number Of Electrons 7 rows the atomic binding energy derives from the electromagnetic interaction of the electrons with the nucleus, mediated by. the definition of the binding energy of an atom to a solid is the difference between the total energy of the solid without the atom. the photoelectron spectrum is a plot of the relative number of electrons emitted. Binding Energy Vs Relative Number Of Electrons.
From www.expii.com
Nuclear Binding Energy — Definition & Overview Expii Binding Energy Vs Relative Number Of Electrons you will find the difference is equal to the electron’s mass to three digits, implying the binding energy is small in comparison. Compare the binding energy of a nucleon in a nucleus to the ionization energy of an electron in an atom. calculate the mass defect and binding energy for a wide range of nuclei. the binding. Binding Energy Vs Relative Number Of Electrons.
From comsol.jp
Differentially Pumped Vacuum Systems for Spectroscopic Imaging COMSOL Binding Energy Vs Relative Number Of Electrons the binding energy is the amount of energy required to eject a particular electron transforming it from a bound state in a specific orbital. the binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. Compare the binding energy of a nucleon in a nucleus to the ionization energy of. Binding Energy Vs Relative Number Of Electrons.
From whatsinsight.org
Nuclear Binding Energy Formula Step By Step Calculation What's Insight Binding Energy Vs Relative Number Of Electrons 7 rows the atomic binding energy derives from the electromagnetic interaction of the electrons with the nucleus, mediated by. the binding energy is the amount of energy required to eject a particular electron transforming it from a bound state in a specific orbital. you will find the difference is equal to the electron’s mass to three digits,. Binding Energy Vs Relative Number Of Electrons.
From commons.wikimedia.org
FileElectron binding energy vs Z.jpg Wikimedia Commons Binding Energy Vs Relative Number Of Electrons calculate the mass defect and binding energy for a wide range of nuclei. the definition of the binding energy of an atom to a solid is the difference between the total energy of the solid without the atom. 7 rows the atomic binding energy derives from the electromagnetic interaction of the electrons with the nucleus, mediated by.. Binding Energy Vs Relative Number Of Electrons.
From www.slideserve.com
PPT Nuclear Binding Energy PowerPoint Presentation, free download Binding Energy Vs Relative Number Of Electrons calculate the mass defect and binding energy for a wide range of nuclei. the relative stability of a nucleus is correlated with its binding energy per nucleon, the total binding energy for the. the binding energy is the amount of energy required to eject a particular electron transforming it from a bound state in a specific orbital.. Binding Energy Vs Relative Number Of Electrons.
From www.numerade.com
SOLVED Relative IC0 electrons 500 0,59 0.0 10.0 Binding energy Binding Energy Vs Relative Number Of Electrons you will find the difference is equal to the electron’s mass to three digits, implying the binding energy is small in comparison. the binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. 7 rows the atomic binding energy derives from the electromagnetic interaction of the electrons with the. Binding Energy Vs Relative Number Of Electrons.
From www.globalsino.com
3d Elements in Periodic Table Binding Energy Vs Relative Number Of Electrons calculate the mass defect and binding energy for a wide range of nuclei. Use a graph of binding energy per nucleon (ben) versus mass number (a) graph to assess the relative stability of a nucleus. the relative stability of a nucleus is correlated with its binding energy per nucleon, the total binding energy for the. the binding. Binding Energy Vs Relative Number Of Electrons.
From large.stanford.edu
Chemical Versus Nuclear Reactions Binding Energy Vs Relative Number Of Electrons the relative stability of a nucleus is correlated with its binding energy per nucleon, the total binding energy for the. calculate the mass defect and binding energy for a wide range of nuclei. the binding energy is the amount of energy required to eject a particular electron transforming it from a bound state in a specific orbital.. Binding Energy Vs Relative Number Of Electrons.
From www.youtube.com
Binding Energy per Nucleon and Stability IB Physics YouTube Binding Energy Vs Relative Number Of Electrons the definition of the binding energy of an atom to a solid is the difference between the total energy of the solid without the atom. Compare the binding energy of a nucleon in a nucleus to the ionization energy of an electron in an atom. the relative stability of a nucleus is correlated with its binding energy per. Binding Energy Vs Relative Number Of Electrons.
From www.researchgate.net
Binding energy per particle as calculated in Equation 2. The Binding Energy Vs Relative Number Of Electrons 7 rows the atomic binding energy derives from the electromagnetic interaction of the electrons with the nucleus, mediated by. you will find the difference is equal to the electron’s mass to three digits, implying the binding energy is small in comparison. the photoelectron spectrum is a plot of the relative number of electrons emitted versus their ionization. Binding Energy Vs Relative Number Of Electrons.