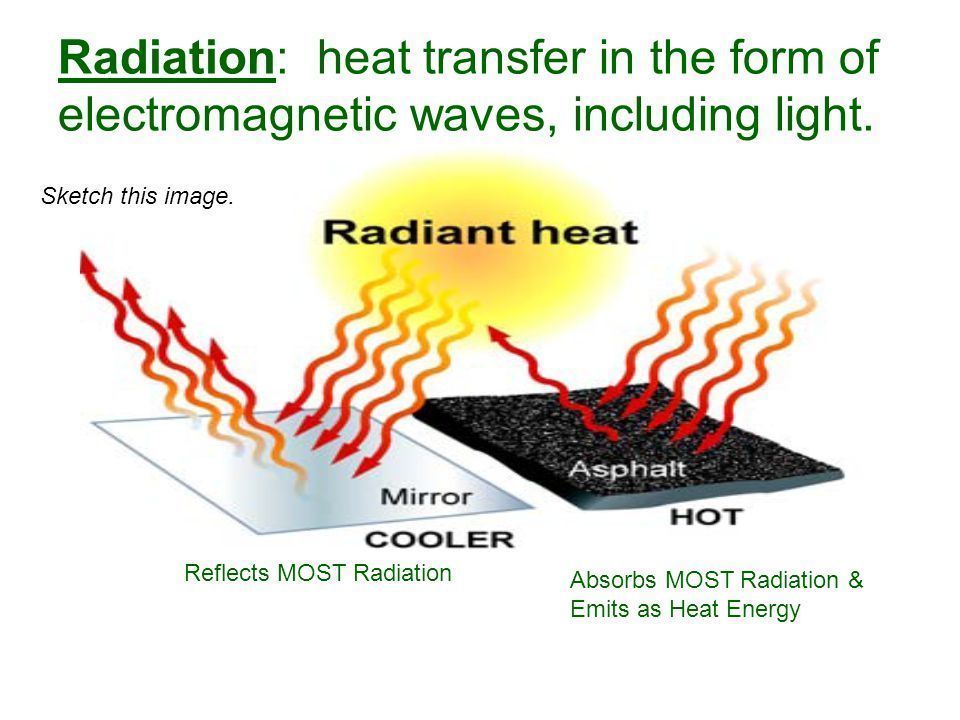
How Does Heat Absorption Work . Objects which take in heat radiation are called absorbers of heat. Heat absorption refers to the process by which a substance takes in thermal energy from its surroundings, causing an increase in its internal. The sun radiates heat energy which travels to earth through the vacuum of space. The symbol c stands for specific heat, and depends on the material and phase. Q = mc ∆ t. What are the factors affecting the amount of heat. Calculate heat absorption using the formula: What are good and bad absorbers of heat? For example, atoms release energy when chemical. Q means the heat absorbed, m is the mass of the substance absorbing heat, c is the specific heat capacity and. While conduction, convection, and radiation are the three modes of heat transfer, other processes absorb and release heat. Heat transferred from the burner of a stove through the bottom of a pan to food in the pan is transferred by conduction. How does the heat travel between objects? The specific heat is the amount of heat necessary to change.
from www.goconqr.com
Heat transferred from the burner of a stove through the bottom of a pan to food in the pan is transferred by conduction. Q means the heat absorbed, m is the mass of the substance absorbing heat, c is the specific heat capacity and. What are the factors affecting the amount of heat. What are good and bad absorbers of heat? Objects which take in heat radiation are called absorbers of heat. The sun radiates heat energy which travels to earth through the vacuum of space. How does the heat travel between objects? The symbol c stands for specific heat, and depends on the material and phase. While conduction, convection, and radiation are the three modes of heat transfer, other processes absorb and release heat. Heat absorption refers to the process by which a substance takes in thermal energy from its surroundings, causing an increase in its internal.
heat transfer conduction, convection, radiation Flashcards
How Does Heat Absorption Work What are the factors affecting the amount of heat. For example, atoms release energy when chemical. Q = mc ∆ t. Q means the heat absorbed, m is the mass of the substance absorbing heat, c is the specific heat capacity and. What are good and bad absorbers of heat? The symbol c stands for specific heat, and depends on the material and phase. Calculate heat absorption using the formula: The specific heat is the amount of heat necessary to change. What are the factors affecting the amount of heat. Heat absorption refers to the process by which a substance takes in thermal energy from its surroundings, causing an increase in its internal. Heat transferred from the burner of a stove through the bottom of a pan to food in the pan is transferred by conduction. While conduction, convection, and radiation are the three modes of heat transfer, other processes absorb and release heat. Objects which take in heat radiation are called absorbers of heat. How does the heat travel between objects? The sun radiates heat energy which travels to earth through the vacuum of space.
From www.youtube.com
Radiation Absorption and Emission Learn All About Temperature & Heat How Does Heat Absorption Work The symbol c stands for specific heat, and depends on the material and phase. Calculate heat absorption using the formula: What are good and bad absorbers of heat? Objects which take in heat radiation are called absorbers of heat. Heat absorption refers to the process by which a substance takes in thermal energy from its surroundings, causing an increase in. How Does Heat Absorption Work.
From www.slideserve.com
PPT Essentials of Fire Fighting , 5 th Edition PowerPoint How Does Heat Absorption Work How does the heat travel between objects? Objects which take in heat radiation are called absorbers of heat. The symbol c stands for specific heat, and depends on the material and phase. Q = mc ∆ t. Heat absorption refers to the process by which a substance takes in thermal energy from its surroundings, causing an increase in its internal.. How Does Heat Absorption Work.
From jtrainfitness.blogspot.com
JTRAIN FITNESS December 2013 How Does Heat Absorption Work Q means the heat absorbed, m is the mass of the substance absorbing heat, c is the specific heat capacity and. The symbol c stands for specific heat, and depends on the material and phase. For example, atoms release energy when chemical. Q = mc ∆ t. The specific heat is the amount of heat necessary to change. Heat transferred. How Does Heat Absorption Work.
From www.youtube.com
Week 1 16. How to calculate the heat absorbed when a substance How Does Heat Absorption Work Heat transferred from the burner of a stove through the bottom of a pan to food in the pan is transferred by conduction. What are the factors affecting the amount of heat. Objects which take in heat radiation are called absorbers of heat. The sun radiates heat energy which travels to earth through the vacuum of space. Q means the. How Does Heat Absorption Work.
From www.slideserve.com
PPT PHYSICAL SCIENCE Temperature and Heat PowerPoint Presentation How Does Heat Absorption Work How does the heat travel between objects? Heat absorption refers to the process by which a substance takes in thermal energy from its surroundings, causing an increase in its internal. For example, atoms release energy when chemical. Heat transferred from the burner of a stove through the bottom of a pan to food in the pan is transferred by conduction.. How Does Heat Absorption Work.
From www.slideserve.com
PPT Chapters 19, 20 Temperature, Heat, and the First Law of How Does Heat Absorption Work What are good and bad absorbers of heat? For example, atoms release energy when chemical. The specific heat is the amount of heat necessary to change. Calculate heat absorption using the formula: How does the heat travel between objects? Heat transferred from the burner of a stove through the bottom of a pan to food in the pan is transferred. How Does Heat Absorption Work.
From www.slideserve.com
PPT Chapter 25.1 “ Factors that Affect Climate ” PowerPoint How Does Heat Absorption Work How does the heat travel between objects? For example, atoms release energy when chemical. Q = mc ∆ t. The specific heat is the amount of heat necessary to change. Objects which take in heat radiation are called absorbers of heat. While conduction, convection, and radiation are the three modes of heat transfer, other processes absorb and release heat. The. How Does Heat Absorption Work.
From www.goconqr.com
heat transfer conduction, convection, radiation Flashcards How Does Heat Absorption Work Q = mc ∆ t. Heat transferred from the burner of a stove through the bottom of a pan to food in the pan is transferred by conduction. For example, atoms release energy when chemical. The sun radiates heat energy which travels to earth through the vacuum of space. What are good and bad absorbers of heat? The symbol c. How Does Heat Absorption Work.
From www.slideserve.com
PPT Fire Streams PowerPoint Presentation, free download ID2203406 How Does Heat Absorption Work Heat absorption refers to the process by which a substance takes in thermal energy from its surroundings, causing an increase in its internal. Heat transferred from the burner of a stove through the bottom of a pan to food in the pan is transferred by conduction. The specific heat is the amount of heat necessary to change. How does the. How Does Heat Absorption Work.
From www.britannica.com
Light Emission and absorption processes Britannica How Does Heat Absorption Work Heat transferred from the burner of a stove through the bottom of a pan to food in the pan is transferred by conduction. Objects which take in heat radiation are called absorbers of heat. What are good and bad absorbers of heat? What are the factors affecting the amount of heat. How does the heat travel between objects? The sun. How Does Heat Absorption Work.
From www.slideserve.com
PPT CHAPTER 7 HEAT PowerPoint Presentation, free download ID2090526 How Does Heat Absorption Work What are the factors affecting the amount of heat. Calculate heat absorption using the formula: The sun radiates heat energy which travels to earth through the vacuum of space. How does the heat travel between objects? What are good and bad absorbers of heat? The symbol c stands for specific heat, and depends on the material and phase. While conduction,. How Does Heat Absorption Work.
From www.slideserve.com
PPT Essentials of Fire Fighting , 5 th Edition PowerPoint How Does Heat Absorption Work How does the heat travel between objects? What are good and bad absorbers of heat? For example, atoms release energy when chemical. While conduction, convection, and radiation are the three modes of heat transfer, other processes absorb and release heat. The sun radiates heat energy which travels to earth through the vacuum of space. The specific heat is the amount. How Does Heat Absorption Work.
From www.slideserve.com
PPT The Absorption of Heat by Solids and Liquids PowerPoint How Does Heat Absorption Work What are good and bad absorbers of heat? Heat absorption refers to the process by which a substance takes in thermal energy from its surroundings, causing an increase in its internal. Q means the heat absorbed, m is the mass of the substance absorbing heat, c is the specific heat capacity and. The symbol c stands for specific heat, and. How Does Heat Absorption Work.
From www.slideserve.com
PPT Fire Streams PowerPoint Presentation, free download ID2203406 How Does Heat Absorption Work The specific heat is the amount of heat necessary to change. For example, atoms release energy when chemical. What are the factors affecting the amount of heat. Heat transferred from the burner of a stove through the bottom of a pan to food in the pan is transferred by conduction. Calculate heat absorption using the formula: Q means the heat. How Does Heat Absorption Work.
From hvacmasterhub.com
How does an absorption heat pump work? HVAC Knowledge Base How Does Heat Absorption Work While conduction, convection, and radiation are the three modes of heat transfer, other processes absorb and release heat. Heat transferred from the burner of a stove through the bottom of a pan to food in the pan is transferred by conduction. The symbol c stands for specific heat, and depends on the material and phase. The sun radiates heat energy. How Does Heat Absorption Work.
From www.researchgate.net
Schematic diagram of an absorption cooling system with heat supply by How Does Heat Absorption Work The sun radiates heat energy which travels to earth through the vacuum of space. For example, atoms release energy when chemical. Heat transferred from the burner of a stove through the bottom of a pan to food in the pan is transferred by conduction. What are the factors affecting the amount of heat. What are good and bad absorbers of. How Does Heat Absorption Work.
From www.deltat.com
Three Ways To Transfer Heat Conduction Convection Radiation Delta T How Does Heat Absorption Work While conduction, convection, and radiation are the three modes of heat transfer, other processes absorb and release heat. Calculate heat absorption using the formula: Q means the heat absorbed, m is the mass of the substance absorbing heat, c is the specific heat capacity and. The symbol c stands for specific heat, and depends on the material and phase. Objects. How Does Heat Absorption Work.
From www.researchgate.net
Heat of absorption of CO 2 with 3.0 M AMP + 0.5 M PZ Download How Does Heat Absorption Work Q means the heat absorbed, m is the mass of the substance absorbing heat, c is the specific heat capacity and. Calculate heat absorption using the formula: The symbol c stands for specific heat, and depends on the material and phase. The specific heat is the amount of heat necessary to change. Heat absorption refers to the process by which. How Does Heat Absorption Work.
From www.climate-encyclopedia.com
Absorption Climate Encyclopedia How Does Heat Absorption Work Calculate heat absorption using the formula: What are the factors affecting the amount of heat. Q means the heat absorbed, m is the mass of the substance absorbing heat, c is the specific heat capacity and. While conduction, convection, and radiation are the three modes of heat transfer, other processes absorb and release heat. For example, atoms release energy when. How Does Heat Absorption Work.
From www.pinterest.com
Latent Heat the heat energy that must be absorbed when a substance How Does Heat Absorption Work Heat transferred from the burner of a stove through the bottom of a pan to food in the pan is transferred by conduction. The specific heat is the amount of heat necessary to change. Objects which take in heat radiation are called absorbers of heat. What are the factors affecting the amount of heat. How does the heat travel between. How Does Heat Absorption Work.
From www.slideserve.com
PPT Pressure Relief PowerPoint Presentation ID214314 How Does Heat Absorption Work While conduction, convection, and radiation are the three modes of heat transfer, other processes absorb and release heat. Calculate heat absorption using the formula: How does the heat travel between objects? Heat absorption refers to the process by which a substance takes in thermal energy from its surroundings, causing an increase in its internal. Heat transferred from the burner of. How Does Heat Absorption Work.
From www.nagwa.com
Question Video Understanding How Heat Absorption and Emission Relates How Does Heat Absorption Work Heat absorption refers to the process by which a substance takes in thermal energy from its surroundings, causing an increase in its internal. Calculate heat absorption using the formula: How does the heat travel between objects? For example, atoms release energy when chemical. Q means the heat absorbed, m is the mass of the substance absorbing heat, c is the. How Does Heat Absorption Work.
From www.slideserve.com
PPT The Absorption of Heat by Solids and Liquids PowerPoint How Does Heat Absorption Work The symbol c stands for specific heat, and depends on the material and phase. Heat transferred from the burner of a stove through the bottom of a pan to food in the pan is transferred by conduction. Calculate heat absorption using the formula: Heat absorption refers to the process by which a substance takes in thermal energy from its surroundings,. How Does Heat Absorption Work.
From www.slideserve.com
PPT Heat Energy Transfer PowerPoint Presentation, free download ID How Does Heat Absorption Work What are the factors affecting the amount of heat. Heat absorption refers to the process by which a substance takes in thermal energy from its surroundings, causing an increase in its internal. For example, atoms release energy when chemical. What are good and bad absorbers of heat? Q = mc ∆ t. Q means the heat absorbed, m is the. How Does Heat Absorption Work.
From www.roburheatpumps.co.uk
What is Gas Absorption? Robur Heat Pumps How Does Heat Absorption Work The symbol c stands for specific heat, and depends on the material and phase. Q = mc ∆ t. Objects which take in heat radiation are called absorbers of heat. The sun radiates heat energy which travels to earth through the vacuum of space. What are good and bad absorbers of heat? Calculate heat absorption using the formula: Heat transferred. How Does Heat Absorption Work.
From www.slideserve.com
PPT Temperature and the Atmosphere PowerPoint Presentation, free How Does Heat Absorption Work How does the heat travel between objects? The symbol c stands for specific heat, and depends on the material and phase. Objects which take in heat radiation are called absorbers of heat. Calculate heat absorption using the formula: What are good and bad absorbers of heat? Heat absorption refers to the process by which a substance takes in thermal energy. How Does Heat Absorption Work.
From www.ces.fau.edu
Climate Science Investigations South Florida Energy The Driver of How Does Heat Absorption Work Heat absorption refers to the process by which a substance takes in thermal energy from its surroundings, causing an increase in its internal. The specific heat is the amount of heat necessary to change. The symbol c stands for specific heat, and depends on the material and phase. Objects which take in heat radiation are called absorbers of heat. Q. How Does Heat Absorption Work.
From www.slideserve.com
PPT HEATING & COOLING EQUIPMENT PowerPoint Presentation, free How Does Heat Absorption Work While conduction, convection, and radiation are the three modes of heat transfer, other processes absorb and release heat. The symbol c stands for specific heat, and depends on the material and phase. Objects which take in heat radiation are called absorbers of heat. For example, atoms release energy when chemical. What are good and bad absorbers of heat? The specific. How Does Heat Absorption Work.
From www.slideserve.com
PPT Thermal Energy PowerPoint Presentation, free download ID1563828 How Does Heat Absorption Work For example, atoms release energy when chemical. Heat absorption refers to the process by which a substance takes in thermal energy from its surroundings, causing an increase in its internal. What are the factors affecting the amount of heat. Q = mc ∆ t. The symbol c stands for specific heat, and depends on the material and phase. How does. How Does Heat Absorption Work.
From www.slideserve.com
PPT The Absorption of Heat by Solids and Liquids PowerPoint How Does Heat Absorption Work The sun radiates heat energy which travels to earth through the vacuum of space. Heat absorption refers to the process by which a substance takes in thermal energy from its surroundings, causing an increase in its internal. What are the factors affecting the amount of heat. Objects which take in heat radiation are called absorbers of heat. The specific heat. How Does Heat Absorption Work.
From general.chemistrysteps.com
What is Enthalpy Chemistry Steps How Does Heat Absorption Work For example, atoms release energy when chemical. The specific heat is the amount of heat necessary to change. Q = mc ∆ t. Heat transferred from the burner of a stove through the bottom of a pan to food in the pan is transferred by conduction. While conduction, convection, and radiation are the three modes of heat transfer, other processes. How Does Heat Absorption Work.
From www.dreamstime.com
Heat Energy As Convection, Conduction and Radiation, Physics Science How Does Heat Absorption Work The specific heat is the amount of heat necessary to change. Heat transferred from the burner of a stove through the bottom of a pan to food in the pan is transferred by conduction. What are the factors affecting the amount of heat. Q = mc ∆ t. The symbol c stands for specific heat, and depends on the material. How Does Heat Absorption Work.
From www.engineering-society.com
Absorption Chiller, How it works working principle hvac How Does Heat Absorption Work The specific heat is the amount of heat necessary to change. How does the heat travel between objects? The sun radiates heat energy which travels to earth through the vacuum of space. Objects which take in heat radiation are called absorbers of heat. Calculate heat absorption using the formula: Heat absorption refers to the process by which a substance takes. How Does Heat Absorption Work.
From www.slideserve.com
PPT KS4 Heat transfer PowerPoint Presentation, free download ID3664069 How Does Heat Absorption Work Q means the heat absorbed, m is the mass of the substance absorbing heat, c is the specific heat capacity and. The sun radiates heat energy which travels to earth through the vacuum of space. The symbol c stands for specific heat, and depends on the material and phase. How does the heat travel between objects? Objects which take in. How Does Heat Absorption Work.
From www.slideserve.com
PPT The Absorption of Heat by Solids and Liquids PowerPoint How Does Heat Absorption Work Objects which take in heat radiation are called absorbers of heat. What are good and bad absorbers of heat? Q = mc ∆ t. Heat transferred from the burner of a stove through the bottom of a pan to food in the pan is transferred by conduction. Q means the heat absorbed, m is the mass of the substance absorbing. How Does Heat Absorption Work.