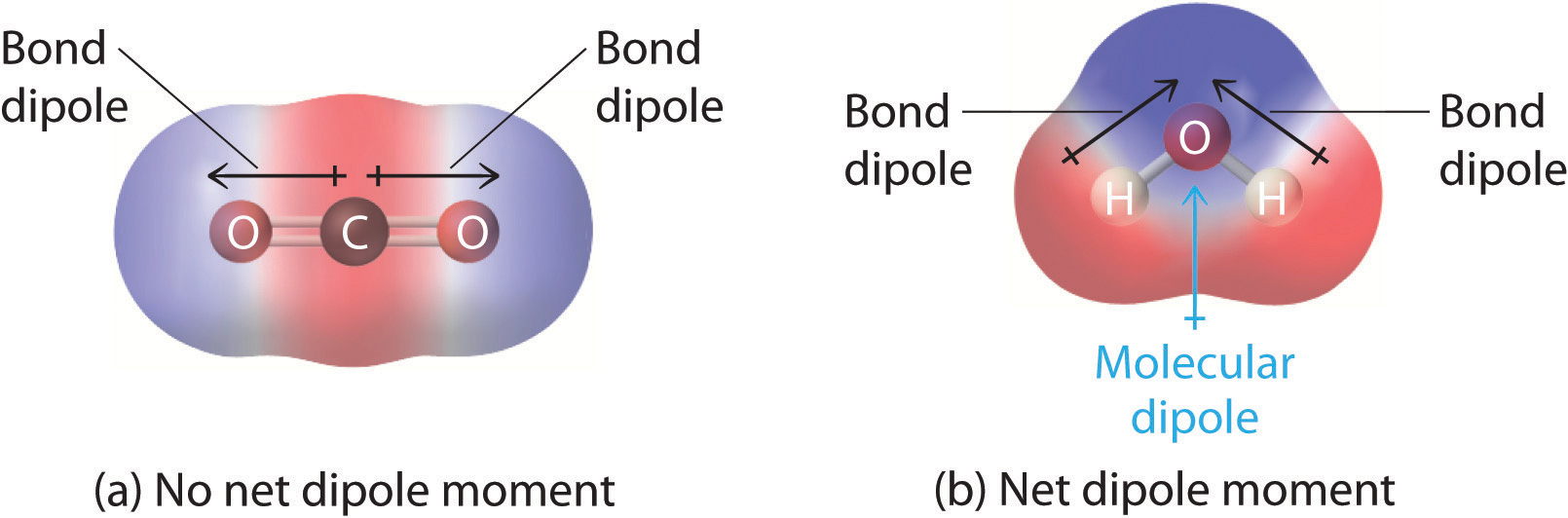
What Does Polarized Mean In Chemistry . In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a. The overall polarity of a molecule has an impact on the behavior of the molecule itself. Typically the electron cloud will belong to an. In contrast, large atoms with many electrons, such as negative ions with excess electrons, are easily polarized. Polarizability is a measure of how easily an electron cloud is distorted by an electric field. Polarity, in chemical bonding, the distribution of electrical charge over the atoms joined by the bond. Polar molecules tend to have higher. The ability of a cation to distort an anion is known as its polarization power and the tendency of the anion to become polarized by. While bonds between identical atoms such as two of.
from chem.libretexts.org
Typically the electron cloud will belong to an. Polarizability is a measure of how easily an electron cloud is distorted by an electric field. In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a. The overall polarity of a molecule has an impact on the behavior of the molecule itself. While bonds between identical atoms such as two of. Polarity, in chemical bonding, the distribution of electrical charge over the atoms joined by the bond. Polar molecules tend to have higher. In contrast, large atoms with many electrons, such as negative ions with excess electrons, are easily polarized. The ability of a cation to distort an anion is known as its polarization power and the tendency of the anion to become polarized by.
2.2 Polar Covalent Bonds Dipole Moments Chemistry LibreTexts
What Does Polarized Mean In Chemistry In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a. Polarizability is a measure of how easily an electron cloud is distorted by an electric field. In contrast, large atoms with many electrons, such as negative ions with excess electrons, are easily polarized. Typically the electron cloud will belong to an. Polar molecules tend to have higher. In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a. The overall polarity of a molecule has an impact on the behavior of the molecule itself. Polarity, in chemical bonding, the distribution of electrical charge over the atoms joined by the bond. While bonds between identical atoms such as two of. The ability of a cation to distort an anion is known as its polarization power and the tendency of the anion to become polarized by.
From biologydictionary.net
Polar Molecule Definition and Examples Biology Dictionary What Does Polarized Mean In Chemistry Polar molecules tend to have higher. Typically the electron cloud will belong to an. The overall polarity of a molecule has an impact on the behavior of the molecule itself. Polarity, in chemical bonding, the distribution of electrical charge over the atoms joined by the bond. In contrast, large atoms with many electrons, such as negative ions with excess electrons,. What Does Polarized Mean In Chemistry.
From chemistnotes.com
Polarization of ion Polarizing power and polarizability Chemistry Notes What Does Polarized Mean In Chemistry In contrast, large atoms with many electrons, such as negative ions with excess electrons, are easily polarized. Polarity, in chemical bonding, the distribution of electrical charge over the atoms joined by the bond. Polarizability is a measure of how easily an electron cloud is distorted by an electric field. The overall polarity of a molecule has an impact on the. What Does Polarized Mean In Chemistry.
From chem.libretexts.org
9.3 Molecular Shape and Molecular Polarity Chemistry LibreTexts What Does Polarized Mean In Chemistry The overall polarity of a molecule has an impact on the behavior of the molecule itself. Polarity, in chemical bonding, the distribution of electrical charge over the atoms joined by the bond. Polarizability is a measure of how easily an electron cloud is distorted by an electric field. While bonds between identical atoms such as two of. Polar molecules tend. What Does Polarized Mean In Chemistry.
From www.thoughtco.com
Definition and Examples of a Polar Bond What Does Polarized Mean In Chemistry Polarity, in chemical bonding, the distribution of electrical charge over the atoms joined by the bond. The overall polarity of a molecule has an impact on the behavior of the molecule itself. Typically the electron cloud will belong to an. The ability of a cation to distort an anion is known as its polarization power and the tendency of the. What Does Polarized Mean In Chemistry.
From www.slideserve.com
PPT Factors that Favour Polarization of Ionic Bond Fajans’ Rules What Does Polarized Mean In Chemistry Typically the electron cloud will belong to an. Polarity, in chemical bonding, the distribution of electrical charge over the atoms joined by the bond. In contrast, large atoms with many electrons, such as negative ions with excess electrons, are easily polarized. While bonds between identical atoms such as two of. The overall polarity of a molecule has an impact on. What Does Polarized Mean In Chemistry.
From www.slideserve.com
PPT Bond Polarity PowerPoint Presentation, free download ID6597592 What Does Polarized Mean In Chemistry Typically the electron cloud will belong to an. While bonds between identical atoms such as two of. Polarity, in chemical bonding, the distribution of electrical charge over the atoms joined by the bond. In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a. Polar molecules tend. What Does Polarized Mean In Chemistry.
From www.chemistrysteps.com
Optical Activity Chemistry Steps What Does Polarized Mean In Chemistry In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a. While bonds between identical atoms such as two of. The overall polarity of a molecule has an impact on the behavior of the molecule itself. Polarity, in chemical bonding, the distribution of electrical charge over the. What Does Polarized Mean In Chemistry.
From www.windward.solutions
How does a polar bond differ from a covalent bond What Does Polarized Mean In Chemistry Typically the electron cloud will belong to an. In contrast, large atoms with many electrons, such as negative ions with excess electrons, are easily polarized. In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a. While bonds between identical atoms such as two of. Polarity, in. What Does Polarized Mean In Chemistry.
From present5.com
POLARIZATION An wave propagates in What Does Polarized Mean In Chemistry Typically the electron cloud will belong to an. The overall polarity of a molecule has an impact on the behavior of the molecule itself. Polarizability is a measure of how easily an electron cloud is distorted by an electric field. The ability of a cation to distort an anion is known as its polarization power and the tendency of the. What Does Polarized Mean In Chemistry.
From www.youtube.com
POLARIZATION FAJAN'S RULE / CHEMICAL BONDING7 YouTube What Does Polarized Mean In Chemistry Polar molecules tend to have higher. Polarizability is a measure of how easily an electron cloud is distorted by an electric field. The overall polarity of a molecule has an impact on the behavior of the molecule itself. The ability of a cation to distort an anion is known as its polarization power and the tendency of the anion to. What Does Polarized Mean In Chemistry.
From www.youtube.com
What is Ion Polarization? And what are the factors affecting it? YouTube What Does Polarized Mean In Chemistry While bonds between identical atoms such as two of. In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a. Polarity, in chemical bonding, the distribution of electrical charge over the atoms joined by the bond. The ability of a cation to distort an anion is known. What Does Polarized Mean In Chemistry.
From www.masterorganicchemistry.com
Optical Rotation, Optical Activity, and Specific Rotation What Does Polarized Mean In Chemistry In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a. In contrast, large atoms with many electrons, such as negative ions with excess electrons, are easily polarized. Polar molecules tend to have higher. Polarity, in chemical bonding, the distribution of electrical charge over the atoms joined. What Does Polarized Mean In Chemistry.
From www.slideserve.com
PPT VSEPR and Chemical Polarity PowerPoint Presentation, free What Does Polarized Mean In Chemistry Polarizability is a measure of how easily an electron cloud is distorted by an electric field. In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a. The overall polarity of a molecule has an impact on the behavior of the molecule itself. In contrast, large atoms. What Does Polarized Mean In Chemistry.
From www.slideserve.com
PPT EE3321 Field Theory PowerPoint Presentation, free What Does Polarized Mean In Chemistry The ability of a cation to distort an anion is known as its polarization power and the tendency of the anion to become polarized by. In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a. Typically the electron cloud will belong to an. In contrast, large. What Does Polarized Mean In Chemistry.
From chem.libretexts.org
2.2 Polar Covalent Bonds Dipole Moments Chemistry LibreTexts What Does Polarized Mean In Chemistry While bonds between identical atoms such as two of. Polar molecules tend to have higher. The ability of a cation to distort an anion is known as its polarization power and the tendency of the anion to become polarized by. Polarizability is a measure of how easily an electron cloud is distorted by an electric field. In contrast, large atoms. What Does Polarized Mean In Chemistry.
From www.slideserve.com
PPT Polarity and Intermolecular Forces PowerPoint Presentation, free What Does Polarized Mean In Chemistry Polarizability is a measure of how easily an electron cloud is distorted by an electric field. The overall polarity of a molecule has an impact on the behavior of the molecule itself. Polar molecules tend to have higher. Polarity, in chemical bonding, the distribution of electrical charge over the atoms joined by the bond. The ability of a cation to. What Does Polarized Mean In Chemistry.
From www.slideserve.com
PPT Waves & the Spectrum PowerPoint What Does Polarized Mean In Chemistry The overall polarity of a molecule has an impact on the behavior of the molecule itself. The ability of a cation to distort an anion is known as its polarization power and the tendency of the anion to become polarized by. While bonds between identical atoms such as two of. Typically the electron cloud will belong to an. Polarizability is. What Does Polarized Mean In Chemistry.
From www.slideserve.com
PPT Chapter 5 PowerPoint Presentation, free download ID3939120 What Does Polarized Mean In Chemistry Typically the electron cloud will belong to an. Polarity, in chemical bonding, the distribution of electrical charge over the atoms joined by the bond. Polar molecules tend to have higher. Polarizability is a measure of how easily an electron cloud is distorted by an electric field. In contrast, large atoms with many electrons, such as negative ions with excess electrons,. What Does Polarized Mean In Chemistry.
From www.britannica.com
Polarization Definition & Types Britannica What Does Polarized Mean In Chemistry Polarity, in chemical bonding, the distribution of electrical charge over the atoms joined by the bond. Polarizability is a measure of how easily an electron cloud is distorted by an electric field. The overall polarity of a molecule has an impact on the behavior of the molecule itself. While bonds between identical atoms such as two of. In chemistry, polarity. What Does Polarized Mean In Chemistry.
From www.youtube.com
Bond Polarity, Electronegativity and Dipole Moment Chemistry Practice What Does Polarized Mean In Chemistry Polar molecules tend to have higher. Polarizability is a measure of how easily an electron cloud is distorted by an electric field. Typically the electron cloud will belong to an. While bonds between identical atoms such as two of. The ability of a cation to distort an anion is known as its polarization power and the tendency of the anion. What Does Polarized Mean In Chemistry.
From chemwiki.ucdavis.edu
Polarizability Chemwiki What Does Polarized Mean In Chemistry The ability of a cation to distort an anion is known as its polarization power and the tendency of the anion to become polarized by. In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a. While bonds between identical atoms such as two of. In contrast,. What Does Polarized Mean In Chemistry.
From www.slideserve.com
PPT Unit 6 Chemical Bonding PowerPoint Presentation, free download What Does Polarized Mean In Chemistry Polarity, in chemical bonding, the distribution of electrical charge over the atoms joined by the bond. Polarizability is a measure of how easily an electron cloud is distorted by an electric field. In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a. Polar molecules tend to. What Does Polarized Mean In Chemistry.
From www.youtube.com
Polarization Of Chemical Bonds YouTube What Does Polarized Mean In Chemistry Polar molecules tend to have higher. Typically the electron cloud will belong to an. The overall polarity of a molecule has an impact on the behavior of the molecule itself. In contrast, large atoms with many electrons, such as negative ions with excess electrons, are easily polarized. While bonds between identical atoms such as two of. In chemistry, polarity is. What Does Polarized Mean In Chemistry.
From www.slideserve.com
PPT Chemical Shapes & Polarity PowerPoint Presentation, free download What Does Polarized Mean In Chemistry In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a. While bonds between identical atoms such as two of. Typically the electron cloud will belong to an. The ability of a cation to distort an anion is known as its polarization power and the tendency of. What Does Polarized Mean In Chemistry.
From www.slideserve.com
PPT Liquids, Solids, and Intermolecular Forces PowerPoint What Does Polarized Mean In Chemistry Polarity, in chemical bonding, the distribution of electrical charge over the atoms joined by the bond. In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a. In contrast, large atoms with many electrons, such as negative ions with excess electrons, are easily polarized. The ability of. What Does Polarized Mean In Chemistry.
From www.youtube.com
Explain the Polarisability of molecule? Raman Spectra Physical What Does Polarized Mean In Chemistry In contrast, large atoms with many electrons, such as negative ions with excess electrons, are easily polarized. Typically the electron cloud will belong to an. The overall polarity of a molecule has an impact on the behavior of the molecule itself. Polarity, in chemical bonding, the distribution of electrical charge over the atoms joined by the bond. While bonds between. What Does Polarized Mean In Chemistry.
From chem.libretexts.org
4.3 Molecular Shape and Molecular Polarity Chemistry LibreTexts What Does Polarized Mean In Chemistry Polarizability is a measure of how easily an electron cloud is distorted by an electric field. In contrast, large atoms with many electrons, such as negative ions with excess electrons, are easily polarized. The overall polarity of a molecule has an impact on the behavior of the molecule itself. In chemistry, polarity is a separation of electric charge leading to. What Does Polarized Mean In Chemistry.
From www.slideserve.com
PPT Chapter 5 PowerPoint Presentation, free download ID888828 What Does Polarized Mean In Chemistry The overall polarity of a molecule has an impact on the behavior of the molecule itself. While bonds between identical atoms such as two of. Polarizability is a measure of how easily an electron cloud is distorted by an electric field. In contrast, large atoms with many electrons, such as negative ions with excess electrons, are easily polarized. The ability. What Does Polarized Mean In Chemistry.
From www.electricity-magnetism.org
Electronic polarization Definition & Characteristics What Does Polarized Mean In Chemistry In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a. The ability of a cation to distort an anion is known as its polarization power and the tendency of the anion to become polarized by. In contrast, large atoms with many electrons, such as negative ions. What Does Polarized Mean In Chemistry.
From scienceinfo.com
Polarization of Ion and Polarizability What Does Polarized Mean In Chemistry While bonds between identical atoms such as two of. Typically the electron cloud will belong to an. The ability of a cation to distort an anion is known as its polarization power and the tendency of the anion to become polarized by. Polar molecules tend to have higher. Polarizability is a measure of how easily an electron cloud is distorted. What Does Polarized Mean In Chemistry.
From socratic.org
What kinds of molecules are polar? + Example What Does Polarized Mean In Chemistry While bonds between identical atoms such as two of. The ability of a cation to distort an anion is known as its polarization power and the tendency of the anion to become polarized by. In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a. Polar molecules. What Does Polarized Mean In Chemistry.
From www.chemicals.co.uk
A Level Chemistry Revision Organic Chemistry Optical Isomerism The What Does Polarized Mean In Chemistry Polarity, in chemical bonding, the distribution of electrical charge over the atoms joined by the bond. Typically the electron cloud will belong to an. Polarizability is a measure of how easily an electron cloud is distorted by an electric field. While bonds between identical atoms such as two of. The ability of a cation to distort an anion is known. What Does Polarized Mean In Chemistry.
From nl.pinterest.com
Why Is Water a Polar Molecule? Hydrogen Atom, Hydrogen Bond, Ionic What Does Polarized Mean In Chemistry Polar molecules tend to have higher. Typically the electron cloud will belong to an. Polarizability is a measure of how easily an electron cloud is distorted by an electric field. The overall polarity of a molecule has an impact on the behavior of the molecule itself. Polarity, in chemical bonding, the distribution of electrical charge over the atoms joined by. What Does Polarized Mean In Chemistry.
From bioswikis.com
What Is Polarization In Chemistry? BiosWikis What Does Polarized Mean In Chemistry In contrast, large atoms with many electrons, such as negative ions with excess electrons, are easily polarized. The overall polarity of a molecule has an impact on the behavior of the molecule itself. In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a. Polarity, in chemical. What Does Polarized Mean In Chemistry.
From chemistnotes.com
Polarization of ion Polarizing power and polarizability Chemistry Notes What Does Polarized Mean In Chemistry In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a. In contrast, large atoms with many electrons, such as negative ions with excess electrons, are easily polarized. The overall polarity of a molecule has an impact on the behavior of the molecule itself. Polarity, in chemical. What Does Polarized Mean In Chemistry.