Calorimeter Heat Capacity Meaning . A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The specific heat is numerically equal to the. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. Its units are joules per degree celsius. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The specific heat (c s) of a substance is the amount of. For example, when an exothermic. The heat capacity (c) of an object is the amount of energy needed to raise its temperature by 1°c; The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. For example, when an exothermic. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The calorimeter heat capacity formula calculates the heat capacity of a calorimeter (cc), which is crucial for.
from www.youtube.com
The heat capacity (c) of an object is the amount of energy needed to raise its temperature by 1°c; The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Its units are joules per degree celsius. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. The specific heat is numerically equal to the. For example, when an exothermic. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
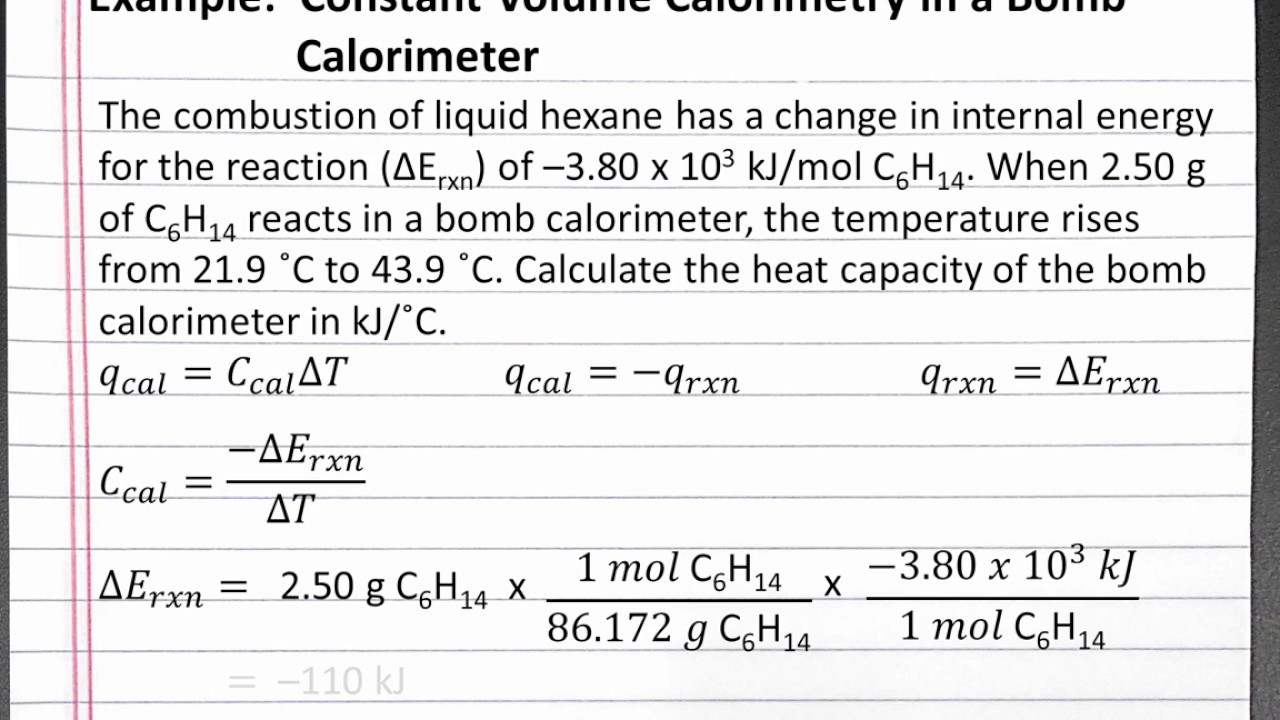
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube
Calorimeter Heat Capacity Meaning A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. The specific heat (c s) of a substance is the amount of. For example, when an exothermic. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. Its units are joules per degree celsius. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The calorimeter heat capacity formula calculates the heat capacity of a calorimeter (cc), which is crucial for. The specific heat is numerically equal to the. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The heat capacity (c) of an object is the amount of energy needed to raise its temperature by 1°c; For example, when an exothermic.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimeter Heat Capacity Meaning The specific heat is numerically equal to the. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. The heat capacity (c) of an object is. Calorimeter Heat Capacity Meaning.
From duntanexhurrard.blogspot.com
DuntanexHurrard Calorimeter Heat Capacity Meaning Its units are joules per degree celsius. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The heat capacity (c) of an object is the amount of energy needed to raise its temperature by 1°c; A calorimeter is a device used to measure the amount. Calorimeter Heat Capacity Meaning.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry Calorimeter Heat Capacity Meaning For example, when an exothermic. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Its units are joules per degree celsius. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The specific heat is numerically equal to the. For. Calorimeter Heat Capacity Meaning.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimeter Heat Capacity Meaning The heat capacity (c) of an object is the amount of energy needed to raise its temperature by 1°c; For example, when an exothermic. For example, when an exothermic. The specific heat is numerically equal to the. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. A calorimeter is. Calorimeter Heat Capacity Meaning.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimeter Heat Capacity Meaning For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or. Its units are joules per. Calorimeter Heat Capacity Meaning.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Calorimeter Heat Capacity Meaning For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Its units are joules per degree celsius. The specific heat is numerically equal to the. A calorimeter. Calorimeter Heat Capacity Meaning.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimeter Heat Capacity Meaning The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. The heat capacity (c) of an object is the amount of energy needed to raise its temperature by 1°c; For example, when an exothermic. For example, when an exothermic. A calorimeter is a device used to. Calorimeter Heat Capacity Meaning.
From www.tec-science.com
Important remarks on the specific heat capacity tecscience Calorimeter Heat Capacity Meaning For example, when an exothermic. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. The specific heat is numerically equal to the. The calorimeter heat capacity formula calculates the heat capacity of a calorimeter (cc), which is crucial for. The symbol c stands for the. Calorimeter Heat Capacity Meaning.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimeter Heat Capacity Meaning A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends. Calorimeter Heat Capacity Meaning.
From ayanahcristien.blogspot.com
20+ Calculating Heat Of Reaction From ConstantPressure Calorimetry Calorimeter Heat Capacity Meaning The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. The specific heat (c s) of a substance is the amount of. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a. Calorimeter Heat Capacity Meaning.
From www.youtube.com
Calorimetry calculation YouTube Calorimeter Heat Capacity Meaning The specific heat (c s) of a substance is the amount of. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Its units are joules per. Calorimeter Heat Capacity Meaning.
From www.numerade.com
SOLVED Use the References to access important values if needed for Calorimeter Heat Capacity Meaning Its units are joules per degree celsius. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. The specific heat is numerically equal to the. For example, when an exothermic. The heat capacity (c) of an object is the amount of energy needed to raise its. Calorimeter Heat Capacity Meaning.
From www.chegg.com
Solved REPORT SHEET EXPERIMENT Heat Of Neutralization 28 Calorimeter Heat Capacity Meaning The specific heat (c s) of a substance is the amount of. For example, when an exothermic. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in. Calorimeter Heat Capacity Meaning.
From haipernews.com
How To Calculate Heat Capacity From Calorimeter Haiper Calorimeter Heat Capacity Meaning The specific heat is numerically equal to the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Its units are joules per degree celsius. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to. Calorimeter Heat Capacity Meaning.
From hxekhffux.blob.core.windows.net
Calorimetry Color Definition at Barbara Armistead blog Calorimeter Heat Capacity Meaning The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Its units are joules per degree celsius. For example, when an exothermic. The heat capacity (c). Calorimeter Heat Capacity Meaning.
From www.studocu.com
Heat Capacity Calorimetry Worksheet answers NAME_________________PER Calorimeter Heat Capacity Meaning Its units are joules per degree celsius. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. The heat capacity (c) of an object is the amount of energy needed to. Calorimeter Heat Capacity Meaning.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Calorimeter Heat Capacity Meaning For example, when an exothermic. Its units are joules per degree celsius. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. A calorimeter. Calorimeter Heat Capacity Meaning.
From www.geeksforgeeks.org
Specific Heat Capacity Definition, Formula, Water Heat Capacity Calorimeter Heat Capacity Meaning A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. A calorimeter is a device used to measure the amount. Calorimeter Heat Capacity Meaning.
From socratic.org
Finding DeltaH given heat released and mass in bomb calorimetry? Socratic Calorimeter Heat Capacity Meaning The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The specific heat is numerically equal to the. The symbol c stands for the specific heat (also. Calorimeter Heat Capacity Meaning.
From evelinrtzamora.blogspot.com
EvelinrtZamora Calorimeter Heat Capacity Meaning The heat capacity (c) of an object is the amount of energy needed to raise its temperature by 1°c; For example, when an exothermic. For example, when an exothermic. The calorimeter heat capacity formula calculates the heat capacity of a calorimeter (cc), which is crucial for. The heat capacity (c) of a body of matter is the quantity of heat. Calorimeter Heat Capacity Meaning.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimeter Heat Capacity Meaning The heat capacity (c) of an object is the amount of energy needed to raise its temperature by 1°c; A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The specific heat (c s) of a substance is the amount of. The specific heat is numerically equal to the. A container. Calorimeter Heat Capacity Meaning.
From www.youtube.com
050 Calorimetry YouTube Calorimeter Heat Capacity Meaning The calorimeter heat capacity formula calculates the heat capacity of a calorimeter (cc), which is crucial for. For example, when an exothermic. The heat capacity (c) of an object is the amount of energy needed to raise its temperature by 1°c; The specific heat (c s) of a substance is the amount of. For example, when an exothermic. A calorimeter. Calorimeter Heat Capacity Meaning.
From www.youtube.com
Heat of Reaction from a Calorimeter (Example) YouTube Calorimeter Heat Capacity Meaning The heat capacity (c) of an object is the amount of energy needed to raise its temperature by 1°c; The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. For example, when an exothermic. The specific heat (c s) of a substance is the amount of.. Calorimeter Heat Capacity Meaning.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Calorimeter Heat Capacity Meaning The calorimeter heat capacity formula calculates the heat capacity of a calorimeter (cc), which is crucial for. For example, when an exothermic. The specific heat is numerically equal to the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of. Calorimeter Heat Capacity Meaning.
From exoczofhf.blob.core.windows.net
Calorimeter Heat Meaning at Rico Padgett blog Calorimeter Heat Capacity Meaning For example, when an exothermic. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The symbol c stands for the specific heat (also called “specific heat. Calorimeter Heat Capacity Meaning.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Calorimeter Heat Capacity Meaning The calorimeter heat capacity formula calculates the heat capacity of a calorimeter (cc), which is crucial for. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Its units are joules per degree celsius. The specific heat (c s) of a substance is the amount of. For example, when an exothermic.. Calorimeter Heat Capacity Meaning.
From studylib.net
Calorimetry_and_Specific_Heat_Capacity Calorimeter Heat Capacity Meaning The calorimeter heat capacity formula calculates the heat capacity of a calorimeter (cc), which is crucial for. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The specific heat (c s) of a substance is the amount of. The symbol c stands for the specific heat (also called “specific heat. Calorimeter Heat Capacity Meaning.
From www.mheducation.com
What is McGraw Hill Virtual Labs? McGraw Hill Higher Education Calorimeter Heat Capacity Meaning For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The calorimeter heat capacity formula calculates the heat capacity of a calorimeter (cc), which is crucial for. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.. Calorimeter Heat Capacity Meaning.
From webapi.bu.edu
⚡ The cold equations analysis. The Cold Equation By Tom Godwin Analysis Calorimeter Heat Capacity Meaning The specific heat is numerically equal to the. For example, when an exothermic. The heat capacity (c) of an object is the amount of energy needed to raise its temperature by 1°c; For example, when an exothermic. Its units are joules per degree celsius. A calorimeter is a device used to measure the amount of heat involved in a chemical. Calorimeter Heat Capacity Meaning.
From worksheetcampusbrows.z13.web.core.windows.net
Equation To Calculate Heat Calorimeter Heat Capacity Meaning Its units are joules per degree celsius. The specific heat (c s) of a substance is the amount of. The heat capacity (c) of an object is the amount of energy needed to raise its temperature by 1°c; The calorimeter heat capacity formula calculates the heat capacity of a calorimeter (cc), which is crucial for. The heat capacity (c) of. Calorimeter Heat Capacity Meaning.
From www.youtube.com
Principle of Calorimetry YouTube Calorimeter Heat Capacity Meaning For example, when an exothermic. Its units are joules per degree celsius. The heat capacity (c) of an object is the amount of energy needed to raise its temperature by 1°c; The specific heat (c s) of a substance is the amount of. A container that prevents heat transfer in or out is called a calorimeter, and the use of. Calorimeter Heat Capacity Meaning.
From answerhappy.com
CALORIMETRY HEAT CAPACITY OF A CALORIMETER NTRODUCTION Lab Data Calorimeter Heat Capacity Meaning For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Its units are joules per degree celsius. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The specific heat is numerically equal to the. The calorimeter. Calorimeter Heat Capacity Meaning.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Calorimeter Heat Capacity Meaning The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature. The calorimeter heat capacity formula calculates the heat capacity of a calorimeter (cc), which is crucial for. For example, when an exothermic. The symbol c stands for the specific heat (also called “specific heat capacity”) and. Calorimeter Heat Capacity Meaning.