Lead Chloride Plus Hydrochloric Acid . During the process, red or brown intermediates can sometimes be observed: Enter an equation of an ionic. Lead (ii) chloride can be made as a white precipitate by adding a solution containing chloride ions to lead (ii) nitrate solution. Lead(ii) is precipitated by sulfide in 0.4 m hydrochloric acid. Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate solution. A balanced equation for the reaction would be. To balance a chemical equation,. Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous solutions of soluble. Lead would react with hydrochloric acid to produce lead chloride and hydrogen gas.
from www.chegg.com
Enter an equation of an ionic. Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate solution. Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Lead would react with hydrochloric acid to produce lead chloride and hydrogen gas. Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous solutions of soluble. To balance a chemical equation,. A balanced equation for the reaction would be. During the process, red or brown intermediates can sometimes be observed: Lead(ii) is precipitated by sulfide in 0.4 m hydrochloric acid.
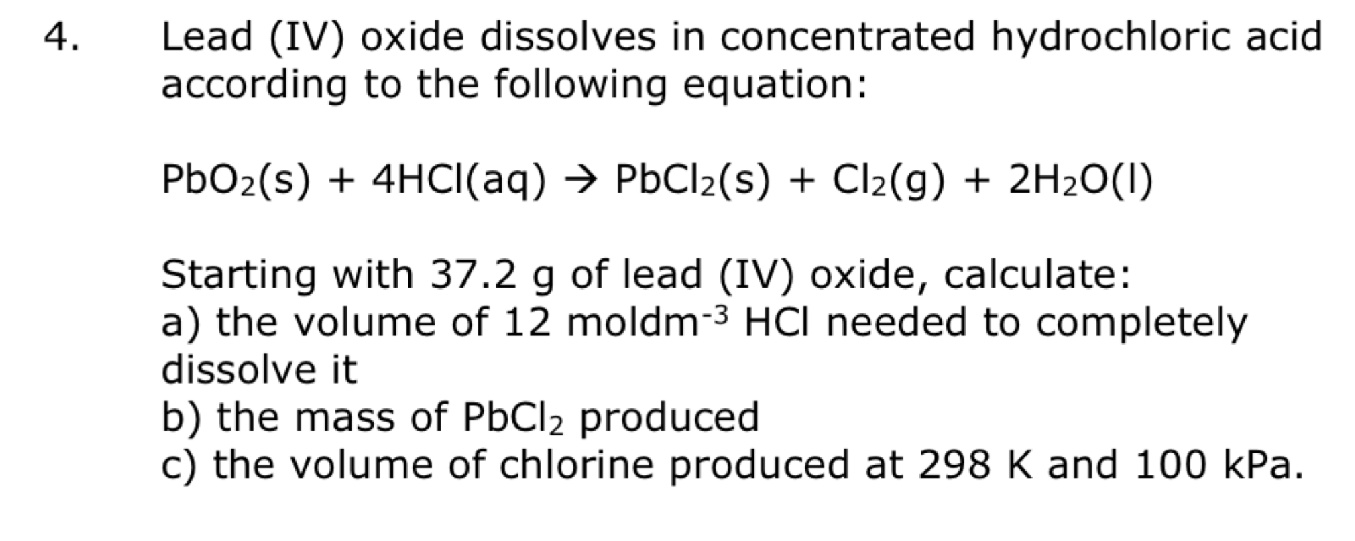
Solved Lead (IV) oxide dissolves in concentrated
Lead Chloride Plus Hydrochloric Acid During the process, red or brown intermediates can sometimes be observed: Lead(ii) is precipitated by sulfide in 0.4 m hydrochloric acid. During the process, red or brown intermediates can sometimes be observed: Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate solution. To balance a chemical equation,. A balanced equation for the reaction would be. Enter an equation of an ionic. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Lead would react with hydrochloric acid to produce lead chloride and hydrogen gas. Lead (ii) chloride can be made as a white precipitate by adding a solution containing chloride ions to lead (ii) nitrate solution. Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous solutions of soluble. Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations.
From www.slideserve.com
PPT PbS HClO 4 HF Zn(OH) 2 HBr sulfur hexafluoride nitrous acid Lead Chloride Plus Hydrochloric Acid A balanced equation for the reaction would be. Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. To balance a chemical equation,. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Lead (ii) chloride can be made as a white precipitate by adding a solution containing chloride. Lead Chloride Plus Hydrochloric Acid.
From www.alamy.com
Hydrochloric Acid High Resolution Stock Photography and Images Alamy Lead Chloride Plus Hydrochloric Acid Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). To balance a chemical equation,. Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous solutions of soluble. Enter an equation of an ionic.. Lead Chloride Plus Hydrochloric Acid.
From www.numerade.com
SOLVED Lead will react with hydrochloric acid to produce lead(II Lead Chloride Plus Hydrochloric Acid A balanced equation for the reaction would be. Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate. Lead Chloride Plus Hydrochloric Acid.
From brainly.in
the reaction between red lead and hydrochloric acid is given as red Lead Chloride Plus Hydrochloric Acid During the process, red or brown intermediates can sometimes be observed: Lead would react with hydrochloric acid to produce lead chloride and hydrogen gas. Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Enter an equation of an ionic. A balanced equation for the reaction would be. Lead (ii) chloride can be made. Lead Chloride Plus Hydrochloric Acid.
From socratic.org
What is the molecular and net ionic equation for the reaction between Lead Chloride Plus Hydrochloric Acid Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Lead(ii) is precipitated by sulfide in 0.4 m hydrochloric acid. Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead. Lead Chloride Plus Hydrochloric Acid.
From www.youtube.com
How to Write the Net Ionic Equation for Ca + HCl = CaCl2 + H2 YouTube Lead Chloride Plus Hydrochloric Acid To balance a chemical equation,. Lead (ii) chloride can be made as a white precipitate by adding a solution containing chloride ions to lead (ii) nitrate solution. Lead(ii) is precipitated by sulfide in 0.4 m hydrochloric acid. A balanced equation for the reaction would be. Enter an equation of an ionic. Lead (ii) chloride, a white precipitate, is formed by. Lead Chloride Plus Hydrochloric Acid.
From rowannewswest.blogspot.com
Balanced Equation of Sodium Carbonate and Hydrochloric Acid Lead Chloride Plus Hydrochloric Acid Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate solution. Lead (ii) chloride can be made as a white precipitate by adding a solution containing chloride ions to lead (ii) nitrate solution. Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations.. Lead Chloride Plus Hydrochloric Acid.
From www.youtube.com
How to write the formula for Hydrochloric acid (HCl) YouTube Lead Chloride Plus Hydrochloric Acid Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate solution. Enter an equation of an ionic. To balance a chemical equation,. Lead would react with hydrochloric acid to produce lead chloride and. Lead Chloride Plus Hydrochloric Acid.
From www.showme.com
ShowMe net ionic equation Lead Chloride Plus Hydrochloric Acid Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous solutions of soluble. During the process, red or brown intermediates can sometimes be observed: Lead (ii) chloride can be made as a white precipitate by adding a solution containing chloride ions to lead (ii) nitrate solution. A balanced equation for the reaction would be. Lead. Lead Chloride Plus Hydrochloric Acid.
From socratic.org
What is the chemical equation for HCl dissolving into water and Lead Chloride Plus Hydrochloric Acid To balance a chemical equation,. Lead (ii) chloride can be made as a white precipitate by adding a solution containing chloride ions to lead (ii) nitrate solution. Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous solutions of soluble. Lead(ii) is precipitated by sulfide in 0.4 m hydrochloric acid. Lead would react with hydrochloric. Lead Chloride Plus Hydrochloric Acid.
From www.chegg.com
Solved Lead (IV) oxide dissolves in concentrated Lead Chloride Plus Hydrochloric Acid A balanced equation for the reaction would be. To balance a chemical equation,. Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate solution. Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Lead (ii) chloride can be made as a white. Lead Chloride Plus Hydrochloric Acid.
From www.alamy.com
Hydrochloric Acid Stock Photos & Hydrochloric Acid Stock Images Alamy Lead Chloride Plus Hydrochloric Acid During the process, red or brown intermediates can sometimes be observed: Enter an equation of an ionic. Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous solutions of soluble. Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. To balance a chemical equation,. Use the calculator below. Lead Chloride Plus Hydrochloric Acid.
From study.com
Hydrogen Chloride vs. Hydrochloric Acid Formula, Properties Lead Chloride Plus Hydrochloric Acid A balanced equation for the reaction would be. Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous solutions of soluble. Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Enter an equation of an ionic. Lead would react with hydrochloric acid to produce lead chloride and hydrogen. Lead Chloride Plus Hydrochloric Acid.
From www.youtube.com
Lead acetate solution is treated with dilute hydrochloric acid to form Lead Chloride Plus Hydrochloric Acid A balanced equation for the reaction would be. During the process, red or brown intermediates can sometimes be observed: To balance a chemical equation,. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous solutions of soluble. Enter an ionic. Lead Chloride Plus Hydrochloric Acid.
From www.sciencephoto.com
Lead in hydrochloric acid Stock Image A500/0623 Science Photo Library Lead Chloride Plus Hydrochloric Acid Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate solution. Lead(ii) is precipitated by sulfide in 0.4 m hydrochloric acid. During the process, red or brown intermediates can sometimes be observed: Lead (ii) chloride can be made as a white precipitate by adding a solution containing chloride ions. Lead Chloride Plus Hydrochloric Acid.
From www.numerade.com
SOLVED The balanced chemical equation for the reaction between calcium Lead Chloride Plus Hydrochloric Acid Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous solutions of soluble. Lead (ii) chloride can be made as a white precipitate by adding a solution containing chloride ions to lead (ii) nitrate solution. Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Use the calculator below. Lead Chloride Plus Hydrochloric Acid.
From www.chegg.com
Solved How many grams of lead chloride are produced from Lead Chloride Plus Hydrochloric Acid Use the calculator below to balance chemical equations and determine the type of reaction (instructions). A balanced equation for the reaction would be. During the process, red or brown intermediates can sometimes be observed: Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous solutions of soluble. Lead (ii) chloride, a white precipitate, is formed. Lead Chloride Plus Hydrochloric Acid.
From byjus.com
Write the neutralization reaction between Hydrochloric acid HCI and Lead Chloride Plus Hydrochloric Acid A balanced equation for the reaction would be. Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate solution. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Lead would react with hydrochloric acid to produce lead chloride and hydrogen gas. To balance. Lead Chloride Plus Hydrochloric Acid.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Lead Chloride Plus Hydrochloric Acid Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous solutions of soluble. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Enter an equation of an ionic. A balanced equation for the reaction would be. Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions. Lead Chloride Plus Hydrochloric Acid.
From www.chemicals.co.uk
The Science Behind Hydrochloric Acid The Chemistry Blog Lead Chloride Plus Hydrochloric Acid To balance a chemical equation,. Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate solution. Enter an equation of an ionic. Lead (ii) chloride can be made as a white precipitate by adding a solution containing chloride ions to lead (ii) nitrate solution. Lead would react with hydrochloric. Lead Chloride Plus Hydrochloric Acid.
From stock.adobe.com
Neutralization Reaction Infographic Diagram with example of Lead Chloride Plus Hydrochloric Acid Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Enter an equation of an ionic. Lead(ii) is precipitated by sulfide in 0.4 m hydrochloric acid. Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate solution. Lead would react with hydrochloric acid. Lead Chloride Plus Hydrochloric Acid.
From www.numerade.com
SOLVED Aqueous hydrochloric acid (HCl) reacts with solid lead(IV Lead Chloride Plus Hydrochloric Acid Use the calculator below to balance chemical equations and determine the type of reaction (instructions). To balance a chemical equation,. A balanced equation for the reaction would be. Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate solution. Lead (ii) chloride can be made as a white precipitate. Lead Chloride Plus Hydrochloric Acid.
From mammothmemory.net
Leads reaction with hydrochloric acid is very slow indeed Lead Chloride Plus Hydrochloric Acid Lead(ii) is precipitated by sulfide in 0.4 m hydrochloric acid. Lead would react with hydrochloric acid to produce lead chloride and hydrogen gas. Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous solutions of soluble. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Lead (ii) chloride can. Lead Chloride Plus Hydrochloric Acid.
From www.youtube.com
Double displacement HCl + Pb(NO3)2 Hydrochloric acid + Lead(ii Lead Chloride Plus Hydrochloric Acid Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous solutions of soluble. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. During the process, red or brown intermediates can sometimes be observed:. Lead Chloride Plus Hydrochloric Acid.
From www.youtube.com
Lead acetate solution is treated with dilute hydrochloric acid to form Lead Chloride Plus Hydrochloric Acid Use the calculator below to balance chemical equations and determine the type of reaction (instructions). During the process, red or brown intermediates can sometimes be observed: Lead (ii) chloride can be made as a white precipitate by adding a solution containing chloride ions to lead (ii) nitrate solution. Enter an ionic equation (solubility states are optional) to calculate its complete. Lead Chloride Plus Hydrochloric Acid.
From www.numerade.com
SOLVED Am asking on how a solid sample of lead ii chloride can be Lead Chloride Plus Hydrochloric Acid During the process, red or brown intermediates can sometimes be observed: Lead (ii) chloride can be made as a white precipitate by adding a solution containing chloride ions to lead (ii) nitrate solution. Enter an equation of an ionic. Lead would react with hydrochloric acid to produce lead chloride and hydrogen gas. Use the calculator below to balance chemical equations. Lead Chloride Plus Hydrochloric Acid.
From www.slideshare.net
5 3 and 5 4 Lead Chloride Plus Hydrochloric Acid A balanced equation for the reaction would be. Lead(ii) is precipitated by sulfide in 0.4 m hydrochloric acid. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Lead (ii) chloride can be made as a white precipitate by adding a solution containing chloride ions to lead (ii) nitrate solution. Enter an equation of an. Lead Chloride Plus Hydrochloric Acid.
From www.sciencephoto.com
Lead in hydrochloric acid Stock Image C043/6238 Science Photo Library Lead Chloride Plus Hydrochloric Acid Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous solutions of soluble. Lead would react with hydrochloric acid to produce lead chloride and hydrogen gas. Lead(ii) is precipitated by sulfide in 0.4 m hydrochloric acid. Enter an equation of. Lead Chloride Plus Hydrochloric Acid.
From www.nagwa.com
Question Video Identifying the Products When a Metal Oxide Reacts with Lead Chloride Plus Hydrochloric Acid Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Lead (ii) chloride can be made as a white precipitate by adding a solution containing chloride ions to lead (ii) nitrate solution. To balance a chemical equation,. Enter an equation of an ionic. Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl,. Lead Chloride Plus Hydrochloric Acid.
From www.numerade.com
SOLVED Lead (II) oxide reacts with hydrochloric acid in a double Lead Chloride Plus Hydrochloric Acid Lead would react with hydrochloric acid to produce lead chloride and hydrogen gas. Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. To balance a chemical equation,. Lead(ii) is precipitated by sulfide in 0.4 m hydrochloric acid. Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous solutions. Lead Chloride Plus Hydrochloric Acid.
From www.tessshebaylo.com
Write The Balanced Chemical Equation For Reaction Of Sodium Carbonate Lead Chloride Plus Hydrochloric Acid Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. During the process, red or brown intermediates can sometimes be observed: Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate solution. Lead would react with hydrochloric acid to produce lead chloride and. Lead Chloride Plus Hydrochloric Acid.
From www.sciencephoto.com
Lead in hydrochloric acid Stock Image C028/0730 Science Photo Library Lead Chloride Plus Hydrochloric Acid Use the calculator below to balance chemical equations and determine the type of reaction (instructions). To balance a chemical equation,. Lead would react with hydrochloric acid to produce lead chloride and hydrogen gas. Lead (ii) chloride, a white precipitate, is formed by adding a chloride ions (in dilute hydrochloric acid) to lead (ii) nitrate solution. Enter an ionic equation (solubility. Lead Chloride Plus Hydrochloric Acid.
From www.alamy.com
Reactivity of metals. Magnesium, zinc, iron and lead in dilute Lead Chloride Plus Hydrochloric Acid A balanced equation for the reaction would be. During the process, red or brown intermediates can sometimes be observed: Lead would react with hydrochloric acid to produce lead chloride and hydrogen gas. Lead (ii) chloride can be made as a white precipitate by adding a solution containing chloride ions to lead (ii) nitrate solution. Use the calculator below to balance. Lead Chloride Plus Hydrochloric Acid.
From www.youtube.com
How to Balance NaOH + HCl = NaCl + H2O (Sodium Hydroxide Plus Lead Chloride Plus Hydrochloric Acid During the process, red or brown intermediates can sometimes be observed: Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous solutions of soluble. Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. Lead (ii) chloride can be made as a white precipitate by adding a solution containing. Lead Chloride Plus Hydrochloric Acid.
From mavink.com
Chemical Formula For Hydrochloric Acid Lead Chloride Plus Hydrochloric Acid Lead(ii) chloride precipitates from solution upon addition of chloride sources (hcl, nacl, kcl) to aqueous solutions of soluble. Enter an ionic equation (solubility states are optional) to calculate its complete and net ionic equations. A balanced equation for the reaction would be. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Lead (ii) chloride,. Lead Chloride Plus Hydrochloric Acid.