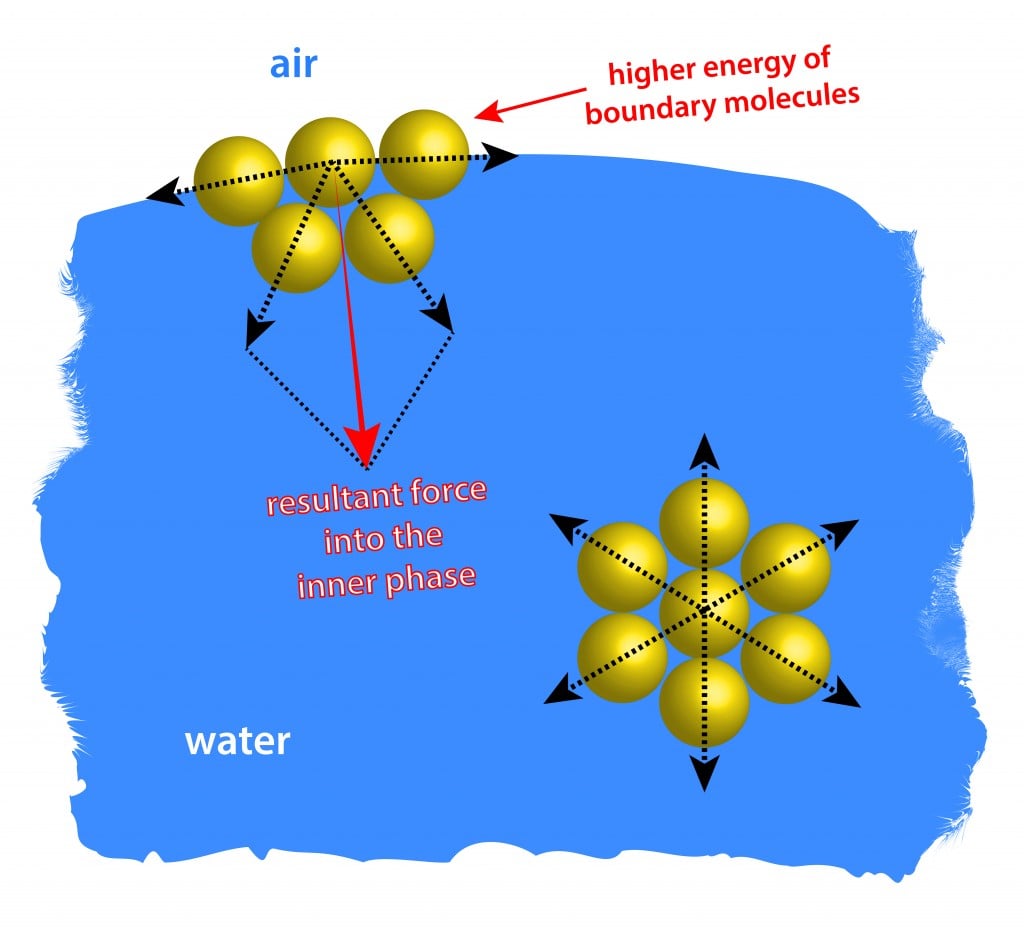
How Does Surface Tension Relate To Polarity . The overall polarity of a molecule has an impact on the behavior of the molecule itself. The stronger the intermolecular interactions, the greater the surface tension. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Learn the surface tension of water. Since these intermolecular forces vary. The attraction of molecules at the surface of a liquid is called surface tension. Check out a few diagrams. How does polarity affect it. Polar molecules tend to have higher. The polarity of water molecules can help explain why water has a strong surface.
from www.scienceabc.com
Surface tension is the energy required to increase the surface area of a liquid by a given amount. The polarity of water molecules can help explain why water has a strong surface. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Learn the surface tension of water. The overall polarity of a molecule has an impact on the behavior of the molecule itself. Check out a few diagrams. Since these intermolecular forces vary. How does polarity affect it. The stronger the intermolecular interactions, the greater the surface tension. Polar molecules tend to have higher.
Surface Tension Definition, Explanation, Examples And Significance
How Does Surface Tension Relate To Polarity The attraction of molecules at the surface of a liquid is called surface tension. Since these intermolecular forces vary. Check out a few diagrams. Learn the surface tension of water. The overall polarity of a molecule has an impact on the behavior of the molecule itself. The attraction of molecules at the surface of a liquid is called surface tension. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Polar molecules tend to have higher. The stronger the intermolecular interactions, the greater the surface tension. The polarity of water molecules can help explain why water has a strong surface. How does polarity affect it. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.
From www.sliderbase.com
Properties of Liquids Presentation Chemistry How Does Surface Tension Relate To Polarity Polar molecules tend to have higher. Check out a few diagrams. The polarity of water molecules can help explain why water has a strong surface. The overall polarity of a molecule has an impact on the behavior of the molecule itself. How does polarity affect it. The stronger the intermolecular interactions, the greater the surface tension. Surface tension is the. How Does Surface Tension Relate To Polarity.
From www.merchantnavydecoded.com
What is Surface tension? Why does surface tension occur? Merchant How Does Surface Tension Relate To Polarity Since these intermolecular forces vary. Check out a few diagrams. The polarity of water molecules can help explain why water has a strong surface. Learn the surface tension of water. The overall polarity of a molecule has an impact on the behavior of the molecule itself. Surface tension is the energy required to increase the surface area of a liquid. How Does Surface Tension Relate To Polarity.
From almerja.com
Surface Tension How Does Surface Tension Relate To Polarity The attraction of molecules at the surface of a liquid is called surface tension. Polar molecules tend to have higher. The overall polarity of a molecule has an impact on the behavior of the molecule itself. Check out a few diagrams. The stronger the intermolecular interactions, the greater the surface tension. Since these intermolecular forces vary. The polarity of water. How Does Surface Tension Relate To Polarity.
From www.slideserve.com
PPT The Nature of Matter PowerPoint Presentation, free download ID How Does Surface Tension Relate To Polarity Learn the surface tension of water. Since these intermolecular forces vary. The attraction of molecules at the surface of a liquid is called surface tension. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension is the energy, or work, required to increase the surface area of a liquid due. How Does Surface Tension Relate To Polarity.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids and Solids PowerPoint How Does Surface Tension Relate To Polarity The stronger the intermolecular interactions, the greater the surface tension. The overall polarity of a molecule has an impact on the behavior of the molecule itself. Polar molecules tend to have higher. How does polarity affect it. Check out a few diagrams. The attraction of molecules at the surface of a liquid is called surface tension. Surface tension is the. How Does Surface Tension Relate To Polarity.
From www.biolinscientific.com
Glossary Knowledge Biolin Scientific How Does Surface Tension Relate To Polarity Learn the surface tension of water. The attraction of molecules at the surface of a liquid is called surface tension. The stronger the intermolecular interactions, the greater the surface tension. Since these intermolecular forces vary. Check out a few diagrams. The polarity of water molecules can help explain why water has a strong surface. Surface tension is the energy, or. How Does Surface Tension Relate To Polarity.
From slideplayer.com
The Properties of Water ppt download How Does Surface Tension Relate To Polarity The overall polarity of a molecule has an impact on the behavior of the molecule itself. The polarity of water molecules can help explain why water has a strong surface. The attraction of molecules at the surface of a liquid is called surface tension. How does polarity affect it. Since these intermolecular forces vary. Surface tension is the energy, or. How Does Surface Tension Relate To Polarity.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance How Does Surface Tension Relate To Polarity The attraction of molecules at the surface of a liquid is called surface tension. How does polarity affect it. Check out a few diagrams. The stronger the intermolecular interactions, the greater the surface tension. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The overall polarity of a molecule. How Does Surface Tension Relate To Polarity.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 How Does Surface Tension Relate To Polarity Polar molecules tend to have higher. The overall polarity of a molecule has an impact on the behavior of the molecule itself. Learn the surface tension of water. The stronger the intermolecular interactions, the greater the surface tension. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension is the. How Does Surface Tension Relate To Polarity.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces How Does Surface Tension Relate To Polarity The attraction of molecules at the surface of a liquid is called surface tension. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The overall polarity of a molecule has an. How Does Surface Tension Relate To Polarity.
From www.elephango.com
Tension in the Water Educational Resources K12 Learning, Physical How Does Surface Tension Relate To Polarity Check out a few diagrams. Surface tension is the energy required to increase the surface area of a liquid by a given amount. How does polarity affect it. The stronger the intermolecular interactions, the greater the surface tension. The overall polarity of a molecule has an impact on the behavior of the molecule itself. Learn the surface tension of water.. How Does Surface Tension Relate To Polarity.
From upberi.com
Surface Tension Definition, Formula, Causes, Examples, and FAQs (2023) How Does Surface Tension Relate To Polarity The overall polarity of a molecule has an impact on the behavior of the molecule itself. Polar molecules tend to have higher. The stronger the intermolecular interactions, the greater the surface tension. Since these intermolecular forces vary. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The attraction of molecules at. How Does Surface Tension Relate To Polarity.
From exoxqkwlq.blob.core.windows.net
How Does Surface Tension Relate To Water at Ron Lowery blog How Does Surface Tension Relate To Polarity Polar molecules tend to have higher. Check out a few diagrams. The polarity of water molecules can help explain why water has a strong surface. The stronger the intermolecular interactions, the greater the surface tension. The overall polarity of a molecule has an impact on the behavior of the molecule itself. Since these intermolecular forces vary. Surface tension is the. How Does Surface Tension Relate To Polarity.
From www.slideserve.com
PPT The Properties of Water PowerPoint Presentation, free download How Does Surface Tension Relate To Polarity Check out a few diagrams. The overall polarity of a molecule has an impact on the behavior of the molecule itself. Surface tension is the energy required to increase the surface area of a liquid by a given amount. How does polarity affect it. Learn the surface tension of water. Polar molecules tend to have higher. Since these intermolecular forces. How Does Surface Tension Relate To Polarity.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it How Does Surface Tension Relate To Polarity How does polarity affect it. The overall polarity of a molecule has an impact on the behavior of the molecule itself. Since these intermolecular forces vary. Polar molecules tend to have higher. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is the energy required to increase. How Does Surface Tension Relate To Polarity.
From www.slideserve.com
PPT Marine Biology Lesson 3 PowerPoint Presentation, free download How Does Surface Tension Relate To Polarity The attraction of molecules at the surface of a liquid is called surface tension. Polar molecules tend to have higher. How does polarity affect it. Since these intermolecular forces vary. The polarity of water molecules can help explain why water has a strong surface. The overall polarity of a molecule has an impact on the behavior of the molecule itself.. How Does Surface Tension Relate To Polarity.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint How Does Surface Tension Relate To Polarity Surface tension is the energy required to increase the surface area of a liquid by a given amount. The overall polarity of a molecule has an impact on the behavior of the molecule itself. The stronger the intermolecular interactions, the greater the surface tension. Since these intermolecular forces vary. The attraction of molecules at the surface of a liquid is. How Does Surface Tension Relate To Polarity.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation, free download ID245553 How Does Surface Tension Relate To Polarity Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The polarity of water molecules can help explain why water has a strong surface. How does polarity affect it. Polar molecules tend. How Does Surface Tension Relate To Polarity.
From upberi.com
Surface Tension Definition, Formula, Causes, Examples, and FAQs (2023) How Does Surface Tension Relate To Polarity Surface tension is the energy required to increase the surface area of a liquid by a given amount. Since these intermolecular forces vary. Learn the surface tension of water. The stronger the intermolecular interactions, the greater the surface tension. Polar molecules tend to have higher. Surface tension is the energy, or work, required to increase the surface area of a. How Does Surface Tension Relate To Polarity.
From www.youtube.com
Surface Tension of Water Explained YouTube How Does Surface Tension Relate To Polarity Check out a few diagrams. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. How does polarity affect it. Since these intermolecular forces vary. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Learn the surface tension of water. The. How Does Surface Tension Relate To Polarity.
From slideplayer.com
The Properties of Water ppt download How Does Surface Tension Relate To Polarity Learn the surface tension of water. The polarity of water molecules can help explain why water has a strong surface. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Check out a few diagrams. Since these intermolecular forces vary. The attraction of molecules at the surface of a liquid. How Does Surface Tension Relate To Polarity.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs How Does Surface Tension Relate To Polarity Since these intermolecular forces vary. The attraction of molecules at the surface of a liquid is called surface tension. How does polarity affect it. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The polarity of water molecules can help explain why water has a strong surface. The stronger. How Does Surface Tension Relate To Polarity.
From www.slideserve.com
PPT Properties of Liquids PowerPoint Presentation, free download ID How Does Surface Tension Relate To Polarity How does polarity affect it. The overall polarity of a molecule has an impact on the behavior of the molecule itself. The stronger the intermolecular interactions, the greater the surface tension. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Polar molecules tend to have higher. Surface tension is the energy,. How Does Surface Tension Relate To Polarity.
From www.slideserve.com
PPT Lesson 3 Cohesion, adhesion & surface tension PowerPoint How Does Surface Tension Relate To Polarity The attraction of molecules at the surface of a liquid is called surface tension. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. How does polarity affect it. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Since these intermolecular. How Does Surface Tension Relate To Polarity.
From www.dataphysics-instruments.com
Dispersive & polar parts of the surface energy DataPhysics Instruments How Does Surface Tension Relate To Polarity The stronger the intermolecular interactions, the greater the surface tension. Since these intermolecular forces vary. The overall polarity of a molecule has an impact on the behavior of the molecule itself. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The polarity of water molecules can help explain why. How Does Surface Tension Relate To Polarity.
From www.slideserve.com
PPT Polarity of water PowerPoint Presentation, free download ID2610682 How Does Surface Tension Relate To Polarity Polar molecules tend to have higher. Learn the surface tension of water. Check out a few diagrams. The overall polarity of a molecule has an impact on the behavior of the molecule itself. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The stronger the intermolecular interactions, the greater the surface. How Does Surface Tension Relate To Polarity.
From www.slideserve.com
PPT Marine Biology Lesson 3 PowerPoint Presentation, free download How Does Surface Tension Relate To Polarity The overall polarity of a molecule has an impact on the behavior of the molecule itself. The polarity of water molecules can help explain why water has a strong surface. How does polarity affect it. Check out a few diagrams. The stronger the intermolecular interactions, the greater the surface tension. Learn the surface tension of water. Surface tension is the. How Does Surface Tension Relate To Polarity.
From manualdefecation.z21.web.core.windows.net
How To Explain Surface Tension How Does Surface Tension Relate To Polarity The overall polarity of a molecule has an impact on the behavior of the molecule itself. Learn the surface tension of water. The polarity of water molecules can help explain why water has a strong surface. The stronger the intermolecular interactions, the greater the surface tension. Since these intermolecular forces vary. Surface tension is the energy required to increase the. How Does Surface Tension Relate To Polarity.
From byjus.com
Explain the surface tension phenomenon with examples. How Does Surface Tension Relate To Polarity Since these intermolecular forces vary. Check out a few diagrams. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The overall polarity of a molecule has an impact on the behavior of the molecule itself. Surface tension is the energy required to increase the surface area of a liquid. How Does Surface Tension Relate To Polarity.
From app.emaze.com
POLAR COVALENT BONDS on emaze How Does Surface Tension Relate To Polarity Surface tension is the energy required to increase the surface area of a liquid by a given amount. Polar molecules tend to have higher. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The polarity of water molecules can help explain why water has a strong surface. The stronger. How Does Surface Tension Relate To Polarity.
From www.dreamstime.com
Surface Tension Explanation Vector Illustration Diagram Stock Vector How Does Surface Tension Relate To Polarity Check out a few diagrams. The stronger the intermolecular interactions, the greater the surface tension. The overall polarity of a molecule has an impact on the behavior of the molecule itself. Since these intermolecular forces vary. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is the. How Does Surface Tension Relate To Polarity.
From study.com
Surface Tension Definition, Calculation & Examples Video & Lesson How Does Surface Tension Relate To Polarity The overall polarity of a molecule has an impact on the behavior of the molecule itself. The polarity of water molecules can help explain why water has a strong surface. How does polarity affect it. Check out a few diagrams. The stronger the intermolecular interactions, the greater the surface tension. The attraction of molecules at the surface of a liquid. How Does Surface Tension Relate To Polarity.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit How Does Surface Tension Relate To Polarity The stronger the intermolecular interactions, the greater the surface tension. Since these intermolecular forces vary. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. How does polarity affect it. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The polarity. How Does Surface Tension Relate To Polarity.
From loetunjhi.blob.core.windows.net
Why Does Surface Tension Happen at Stan Stewart blog How Does Surface Tension Relate To Polarity The overall polarity of a molecule has an impact on the behavior of the molecule itself. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The stronger the intermolecular interactions, the greater the surface tension. Learn the surface tension of water. Polar molecules tend to have higher. The polarity. How Does Surface Tension Relate To Polarity.
From civilquery.com
What is Surface Tension Definition, Examples and Tests Civil Query How Does Surface Tension Relate To Polarity The stronger the intermolecular interactions, the greater the surface tension. Check out a few diagrams. Polar molecules tend to have higher. The attraction of molecules at the surface of a liquid is called surface tension. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Since these intermolecular forces vary. The overall. How Does Surface Tension Relate To Polarity.