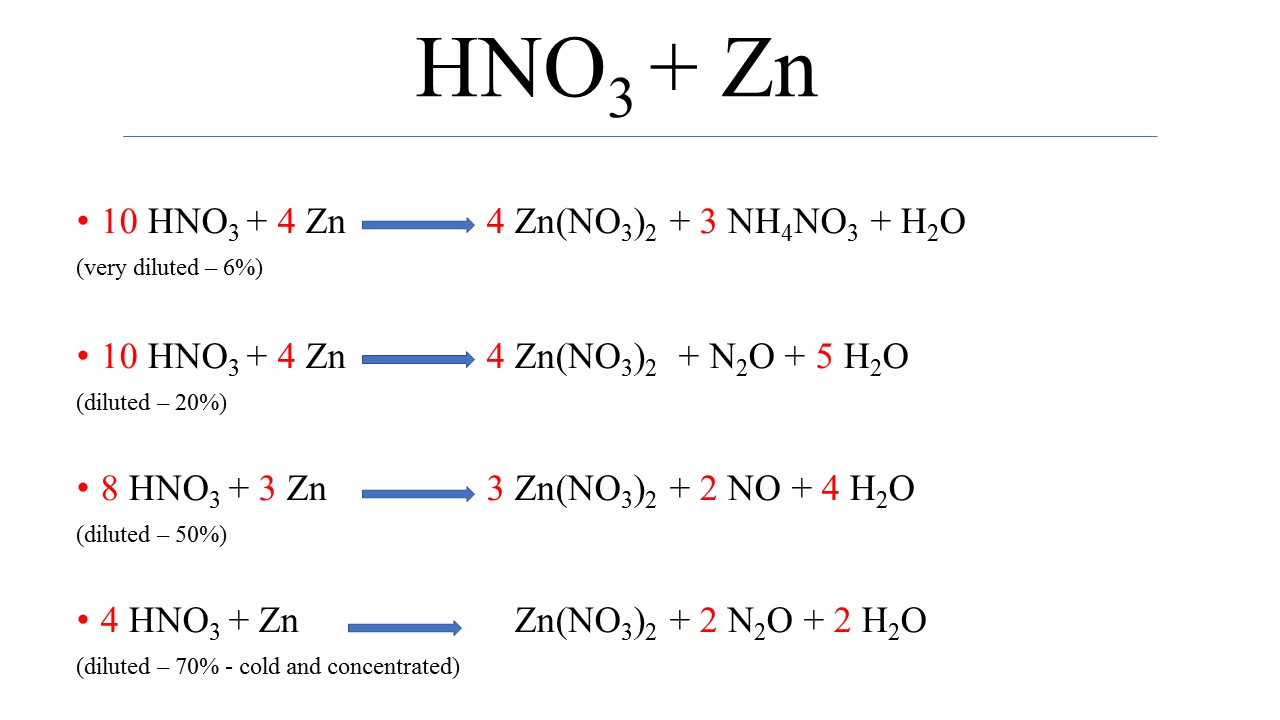
Zinc And Nitric Acid . Hydrochloric acid always produces salts that. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. And you must not not forget the. Reaction of zinc with nitric acid: Using the zinc and dilute sulfuric acid to make hydrogen in the lab. You should convert the reactants and products to ions to identify what actually changes. Zinc reacts steadily with both acids to give colourless solutions of zinc chloride or zinc sulfate together with hydrogen. Magnesium plus nitric acid gives us magnesium nitrate. Calcium plus hydrochloric acid gives us calcium chloride. When zinc zn metal reacts with very dilute nitric acid hno 3, it produces zinc nitrate zn no 3 2, ammonium. Acid + metal → salt + hydrogen. And zinc plus sulphuric acid. This is a traditional way of producing small amounts of hydrogen in the lab. Zinc is a bit slow to react with dilute acids.
from ask.learncbse.in
Acid + metal → salt + hydrogen. And you must not not forget the. Magnesium plus nitric acid gives us magnesium nitrate. Using the zinc and dilute sulfuric acid to make hydrogen in the lab. Calcium plus hydrochloric acid gives us calcium chloride. You should convert the reactants and products to ions to identify what actually changes. Zinc reacts steadily with both acids to give colourless solutions of zinc chloride or zinc sulfate together with hydrogen. Zinc is a bit slow to react with dilute acids. And zinc plus sulphuric acid. When zinc zn metal reacts with very dilute nitric acid hno 3, it produces zinc nitrate zn no 3 2, ammonium.
Balancing the chemical equation CBSE Class 10 Science Learn CBSE Forum
Zinc And Nitric Acid Calcium plus hydrochloric acid gives us calcium chloride. Using the zinc and dilute sulfuric acid to make hydrogen in the lab. This is a traditional way of producing small amounts of hydrogen in the lab. Zinc reacts steadily with both acids to give colourless solutions of zinc chloride or zinc sulfate together with hydrogen. You should convert the reactants and products to ions to identify what actually changes. Hydrochloric acid always produces salts that. Calcium plus hydrochloric acid gives us calcium chloride. Acid + metal → salt + hydrogen. Magnesium plus nitric acid gives us magnesium nitrate. Zinc is a bit slow to react with dilute acids. And zinc plus sulphuric acid. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Reaction of zinc with nitric acid: When zinc zn metal reacts with very dilute nitric acid hno 3, it produces zinc nitrate zn no 3 2, ammonium. And you must not not forget the.
From www.youtube.com
HNO3+Zn=Zn(NO3)2+H2 Balanced EquationNitric acid+Zinc=Zinc nitrate Zinc And Nitric Acid When zinc zn metal reacts with very dilute nitric acid hno 3, it produces zinc nitrate zn no 3 2, ammonium. This is a traditional way of producing small amounts of hydrogen in the lab. Using the zinc and dilute sulfuric acid to make hydrogen in the lab. Acid + metal → salt + hydrogen. Magnesium plus nitric acid gives. Zinc And Nitric Acid.
From www.youtube.com
Nitric acid, Copper and Zinc YouTube Zinc And Nitric Acid Zinc reacts steadily with both acids to give colourless solutions of zinc chloride or zinc sulfate together with hydrogen. Using the zinc and dilute sulfuric acid to make hydrogen in the lab. Reaction of zinc with nitric acid: You should convert the reactants and products to ions to identify what actually changes. This is a traditional way of producing small. Zinc And Nitric Acid.
From www.slideserve.com
PPT Metal Reactions and Reactivity PowerPoint Presentation, free Zinc And Nitric Acid You should convert the reactants and products to ions to identify what actually changes. Reaction of zinc with nitric acid: Magnesium plus nitric acid gives us magnesium nitrate. Zinc reacts steadily with both acids to give colourless solutions of zinc chloride or zinc sulfate together with hydrogen. Using the zinc and dilute sulfuric acid to make hydrogen in the lab.. Zinc And Nitric Acid.
From www.slideserve.com
PPT ACIDS & BASES PowerPoint Presentation, free download ID2732225 Zinc And Nitric Acid Zinc is a bit slow to react with dilute acids. This is a traditional way of producing small amounts of hydrogen in the lab. Zinc reacts steadily with both acids to give colourless solutions of zinc chloride or zinc sulfate together with hydrogen. Calcium plus hydrochloric acid gives us calcium chloride. You should convert the reactants and products to ions. Zinc And Nitric Acid.
From brainly.in
reaction of zinc with nitric acid Brainly.in Zinc And Nitric Acid Zinc is a bit slow to react with dilute acids. And you must not not forget the. This is a traditional way of producing small amounts of hydrogen in the lab. You should convert the reactants and products to ions to identify what actually changes. Hydrochloric acid always produces salts that. And zinc plus sulphuric acid. Calcium plus hydrochloric acid. Zinc And Nitric Acid.
From www.numerade.com
SOLVED 'What salt is formed in the reaction of zinc with nitric acid Zinc And Nitric Acid Magnesium plus nitric acid gives us magnesium nitrate. Hydrochloric acid always produces salts that. Zinc is a bit slow to react with dilute acids. Using the zinc and dilute sulfuric acid to make hydrogen in the lab. Acid + metal → salt + hydrogen. This is a traditional way of producing small amounts of hydrogen in the lab. Zinc reacts. Zinc And Nitric Acid.
From www.youtube.com
Reaction between zinc & Nitric acid 50 YouTube Zinc And Nitric Acid And zinc plus sulphuric acid. Acid + metal → salt + hydrogen. You should convert the reactants and products to ions to identify what actually changes. When zinc zn metal reacts with very dilute nitric acid hno 3, it produces zinc nitrate zn no 3 2, ammonium. And you must not not forget the. Calcium plus hydrochloric acid gives us. Zinc And Nitric Acid.
From www.youtube.com
Zinc metal reaction Nitric acid reaction Zinc in nitric acid Zinc And Nitric Acid Zinc is a bit slow to react with dilute acids. Using the zinc and dilute sulfuric acid to make hydrogen in the lab. Zinc reacts steadily with both acids to give colourless solutions of zinc chloride or zinc sulfate together with hydrogen. This is a traditional way of producing small amounts of hydrogen in the lab. When zinc zn metal. Zinc And Nitric Acid.
From www.youtube.com
How to Balance ZnO + HNO3 = Zn(NO3)2 + H2O (Zinc oxide + Nitric acid Zinc And Nitric Acid You should convert the reactants and products to ions to identify what actually changes. Hydrochloric acid always produces salts that. This is a traditional way of producing small amounts of hydrogen in the lab. Zinc reacts steadily with both acids to give colourless solutions of zinc chloride or zinc sulfate together with hydrogen. Acid + metal → salt + hydrogen.. Zinc And Nitric Acid.
From www.numerade.com
SOLVED Zinc metal reacts with concentrated nitric acid to give zinc Zinc And Nitric Acid And you must not not forget the. This is a traditional way of producing small amounts of hydrogen in the lab. And zinc plus sulphuric acid. Magnesium plus nitric acid gives us magnesium nitrate. Zinc is a bit slow to react with dilute acids. When zinc zn metal reacts with very dilute nitric acid hno 3, it produces zinc nitrate. Zinc And Nitric Acid.
From www.numerade.com
SOLVED 369 of RedoxBalancing Which of the following is the correctly Zinc And Nitric Acid And zinc plus sulphuric acid. Zinc is a bit slow to react with dilute acids. Acid + metal → salt + hydrogen. Zinc reacts steadily with both acids to give colourless solutions of zinc chloride or zinc sulfate together with hydrogen. Using the zinc and dilute sulfuric acid to make hydrogen in the lab. Calcium plus hydrochloric acid gives us. Zinc And Nitric Acid.
From www.youtube.com
How to Write the Net Ionic Equation for Zn + HNO3 = Zn(NO3)2 + H2 YouTube Zinc And Nitric Acid Using the zinc and dilute sulfuric acid to make hydrogen in the lab. Zinc reacts steadily with both acids to give colourless solutions of zinc chloride or zinc sulfate together with hydrogen. And you must not not forget the. Zinc is a bit slow to react with dilute acids. Reaction of zinc with nitric acid: Magnesium plus nitric acid gives. Zinc And Nitric Acid.
From byjus.com
9.Reaction of Zn ,Mg ,Al ,Na, Cu ,Fe , with nitric acid. Balanced Zinc And Nitric Acid When zinc zn metal reacts with very dilute nitric acid hno 3, it produces zinc nitrate zn no 3 2, ammonium. Zinc reacts steadily with both acids to give colourless solutions of zinc chloride or zinc sulfate together with hydrogen. Using the zinc and dilute sulfuric acid to make hydrogen in the lab. Acids will react with reactive metals, such. Zinc And Nitric Acid.
From www.youtube.com
Zinc and Nitric Acid Reaction A Colorful Chemical Reaction Zinc And Nitric Acid Hydrochloric acid always produces salts that. You should convert the reactants and products to ions to identify what actually changes. And you must not not forget the. Magnesium plus nitric acid gives us magnesium nitrate. Zinc reacts steadily with both acids to give colourless solutions of zinc chloride or zinc sulfate together with hydrogen. Acids will react with reactive metals,. Zinc And Nitric Acid.
From www.youtube.com
Write equations for the following reactions zinc and concentrated Zinc And Nitric Acid Reaction of zinc with nitric acid: Magnesium plus nitric acid gives us magnesium nitrate. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. This is a traditional way of producing small amounts of hydrogen in the lab. Using the zinc and dilute sulfuric acid to make hydrogen in the lab. Zinc is. Zinc And Nitric Acid.
From www.numerade.com
SOLVED Nitric acid (HNO3) and zinc react to form zinc nitrate Zinc And Nitric Acid Zinc reacts steadily with both acids to give colourless solutions of zinc chloride or zinc sulfate together with hydrogen. When zinc zn metal reacts with very dilute nitric acid hno 3, it produces zinc nitrate zn no 3 2, ammonium. Magnesium plus nitric acid gives us magnesium nitrate. Reaction of zinc with nitric acid: Acid + metal → salt +. Zinc And Nitric Acid.
From brainly.com
When zinc metal is reacted with nitric acid, ammonium nitrate, zinc Zinc And Nitric Acid Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Calcium plus hydrochloric acid gives us calcium chloride. Acid + metal → salt + hydrogen. Using the zinc and dilute sulfuric acid to make hydrogen in the lab. This is a traditional way of producing small amounts of hydrogen in the lab. Zinc. Zinc And Nitric Acid.
From www.chegg.com
Solved Zinc And Nitric Acid Hydrochloric acid always produces salts that. And you must not not forget the. You should convert the reactants and products to ions to identify what actually changes. Using the zinc and dilute sulfuric acid to make hydrogen in the lab. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Zinc reacts steadily. Zinc And Nitric Acid.
From brainly.in
zinc react with nitric acid to form zinc nitrate and hydrogen gas Zinc And Nitric Acid And zinc plus sulphuric acid. Acid + metal → salt + hydrogen. You should convert the reactants and products to ions to identify what actually changes. Calcium plus hydrochloric acid gives us calcium chloride. And you must not not forget the. When zinc zn metal reacts with very dilute nitric acid hno 3, it produces zinc nitrate zn no 3. Zinc And Nitric Acid.
From www.slideserve.com
PPT pblock elements PowerPoint Presentation, free download ID9468485 Zinc And Nitric Acid You should convert the reactants and products to ions to identify what actually changes. Using the zinc and dilute sulfuric acid to make hydrogen in the lab. And you must not not forget the. Zinc is a bit slow to react with dilute acids. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and. Zinc And Nitric Acid.
From ask.learncbse.in
Balancing the chemical equation CBSE Class 10 Science Learn CBSE Forum Zinc And Nitric Acid And zinc plus sulphuric acid. Using the zinc and dilute sulfuric acid to make hydrogen in the lab. You should convert the reactants and products to ions to identify what actually changes. Zinc reacts steadily with both acids to give colourless solutions of zinc chloride or zinc sulfate together with hydrogen. And you must not not forget the. Magnesium plus. Zinc And Nitric Acid.
From revision.co.zw
The reaction of metals with dilute acids Free ZIMSEC & Cambridge Zinc And Nitric Acid When zinc zn metal reacts with very dilute nitric acid hno 3, it produces zinc nitrate zn no 3 2, ammonium. This is a traditional way of producing small amounts of hydrogen in the lab. Acid + metal → salt + hydrogen. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Using. Zinc And Nitric Acid.
From www.youtube.com
Redox Reaction Zinc with Nitric Acid YouTube Zinc And Nitric Acid Calcium plus hydrochloric acid gives us calcium chloride. Acid + metal → salt + hydrogen. Magnesium plus nitric acid gives us magnesium nitrate. When zinc zn metal reacts with very dilute nitric acid hno 3, it produces zinc nitrate zn no 3 2, ammonium. Reaction of zinc with nitric acid: Zinc reacts steadily with both acids to give colourless solutions. Zinc And Nitric Acid.
From www.youtube.com
Nitric acid and zinc reaction YouTube Zinc And Nitric Acid When zinc zn metal reacts with very dilute nitric acid hno 3, it produces zinc nitrate zn no 3 2, ammonium. Calcium plus hydrochloric acid gives us calcium chloride. Reaction of zinc with nitric acid: You should convert the reactants and products to ions to identify what actually changes. Magnesium plus nitric acid gives us magnesium nitrate. Hydrochloric acid always. Zinc And Nitric Acid.
From www.numerade.com
SOLVEDWrite a balanced net ionic equation for each of the following Zinc And Nitric Acid This is a traditional way of producing small amounts of hydrogen in the lab. Zinc is a bit slow to react with dilute acids. Reaction of zinc with nitric acid: And you must not not forget the. You should convert the reactants and products to ions to identify what actually changes. Magnesium plus nitric acid gives us magnesium nitrate. Calcium. Zinc And Nitric Acid.
From klabxtjsm.blob.core.windows.net
Potassium Hydroxide And Zinc Equation at James Roden blog Zinc And Nitric Acid Calcium plus hydrochloric acid gives us calcium chloride. When zinc zn metal reacts with very dilute nitric acid hno 3, it produces zinc nitrate zn no 3 2, ammonium. Zinc reacts steadily with both acids to give colourless solutions of zinc chloride or zinc sulfate together with hydrogen. This is a traditional way of producing small amounts of hydrogen in. Zinc And Nitric Acid.
From www.youtube.com
What happens when Zinc (Zn) reacts with conc Nitric acid (HNO3)? Zn Zinc And Nitric Acid Zinc is a bit slow to react with dilute acids. Zinc reacts steadily with both acids to give colourless solutions of zinc chloride or zinc sulfate together with hydrogen. Reaction of zinc with nitric acid: Hydrochloric acid always produces salts that. This is a traditional way of producing small amounts of hydrogen in the lab. And zinc plus sulphuric acid.. Zinc And Nitric Acid.
From www.youtube.com
When zinc reacts with very dilute nitric acid it produces YouTube Zinc And Nitric Acid Hydrochloric acid always produces salts that. Using the zinc and dilute sulfuric acid to make hydrogen in the lab. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. When zinc zn metal reacts with very dilute nitric acid hno 3, it produces zinc nitrate zn no 3 2, ammonium. Acid + metal. Zinc And Nitric Acid.
From www.chegg.com
Solved vii) The reaction between zinc and nitric acid. L05 Zinc And Nitric Acid Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Acid + metal → salt + hydrogen. You should convert the reactants and products to ions to identify what actually changes. Reaction of zinc with nitric acid: Zinc is a bit slow to react with dilute acids. Using the zinc and dilute sulfuric. Zinc And Nitric Acid.
From www.slideserve.com
PPT DO NOW!! PowerPoint Presentation, free download ID1560113 Zinc And Nitric Acid And you must not not forget the. Calcium plus hydrochloric acid gives us calcium chloride. When zinc zn metal reacts with very dilute nitric acid hno 3, it produces zinc nitrate zn no 3 2, ammonium. Zinc reacts steadily with both acids to give colourless solutions of zinc chloride or zinc sulfate together with hydrogen. Acids will react with reactive. Zinc And Nitric Acid.
From www.toppr.com
The reaction of zinc with dilute and concentrated nitric acid Zinc And Nitric Acid Hydrochloric acid always produces salts that. Zinc is a bit slow to react with dilute acids. Zinc reacts steadily with both acids to give colourless solutions of zinc chloride or zinc sulfate together with hydrogen. This is a traditional way of producing small amounts of hydrogen in the lab. Calcium plus hydrochloric acid gives us calcium chloride. And you must. Zinc And Nitric Acid.
From edurev.in
The reaction of zinc with dilute and concentrated nitric acid Zinc And Nitric Acid This is a traditional way of producing small amounts of hydrogen in the lab. And you must not not forget the. Using the zinc and dilute sulfuric acid to make hydrogen in the lab. Magnesium plus nitric acid gives us magnesium nitrate. Calcium plus hydrochloric acid gives us calcium chloride. Acids will react with reactive metals, such as magnesium and. Zinc And Nitric Acid.
From www.youtube.com
Reactions of Nitric acid YouTube Zinc And Nitric Acid Reaction of zinc with nitric acid: Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. When zinc zn metal reacts with very dilute nitric acid hno 3, it produces zinc nitrate zn no 3 2, ammonium. And you must not not forget the. And zinc plus sulphuric acid. Hydrochloric acid always produces. Zinc And Nitric Acid.
From www.youtube.com
Equation for Zn(NO3)2 + H2O (Zinc nitrate + Water) YouTube Zinc And Nitric Acid Reaction of zinc with nitric acid: Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Hydrochloric acid always produces salts that. This is a traditional way of producing small amounts of hydrogen in the lab. Magnesium plus nitric acid gives us magnesium nitrate. When zinc zn metal reacts with very dilute nitric. Zinc And Nitric Acid.
From www.slideshare.net
Acids And Bases Zinc And Nitric Acid You should convert the reactants and products to ions to identify what actually changes. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Calcium plus hydrochloric acid gives us calcium chloride. When zinc zn metal reacts with very dilute nitric acid hno 3, it produces zinc nitrate zn no 3 2, ammonium.. Zinc And Nitric Acid.