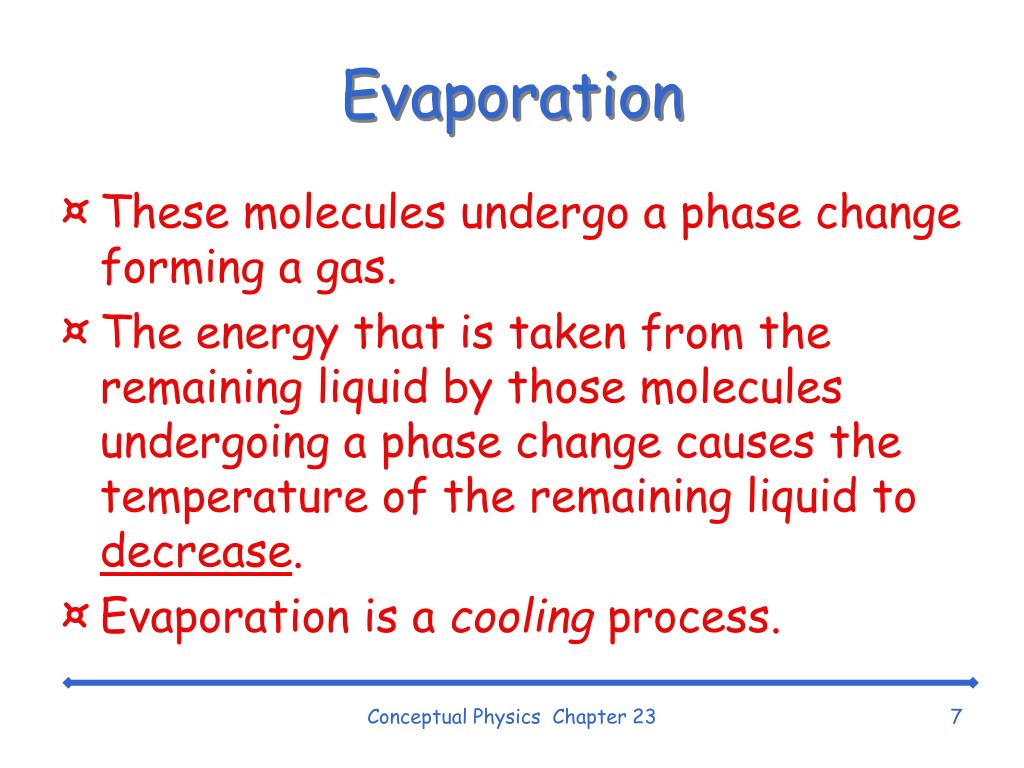
What Is A Evaporation In Phase Change . Vaporization, which is more often referred to as boiling, is the complementary process in which a chemical is converted from the liquid state of. In evaporation, a liquid turns into a gas; A phase change is a physical process in which a substance goes from one phase to another. Changes of state are examples of phase changes, or phase transitions. Evaporation is the opposite of condensation. Evaporation is the process by which molecules undergo the a spontaneous transition from the liquid phase to the gas phase. An interesting feature of the process of cooling the human body by evaporation is that the heat extracted by the evaporation of a gram of. Usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or the boiling point of the substance. The opposite process is condensation. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. A substance melts or freezes at a temperature called its melting point, and boils (evaporates rapidly) or. All phase changes are accompanied by changes in the energy of a system. Because a gas has more energy than a liquid, that means that energy must be provided to a. Vaporization is the phase change that occurs when a substance converts from a liquid to a gas. The melting point is the temperature at which the substance goes from a solid to a liquid (or from a.
from www.slideserve.com
All phase changes are accompanied by changes in the energy of a system. Evaporation is the process by which molecules undergo the a spontaneous transition from the liquid phase to the gas phase. Vaporization is the phase change that occurs when a substance converts from a liquid to a gas. Usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or the boiling point of the substance. Because a gas has more energy than a liquid, that means that energy must be provided to a. In evaporation, a liquid turns into a gas; A substance melts or freezes at a temperature called its melting point, and boils (evaporates rapidly) or. The melting point is the temperature at which the substance goes from a solid to a liquid (or from a. An interesting feature of the process of cooling the human body by evaporation is that the heat extracted by the evaporation of a gram of. Changes of state are examples of phase changes, or phase transitions.
PPT Chapter 23 Changes of Phase PowerPoint Presentation, free
What Is A Evaporation In Phase Change All phase changes are accompanied by changes in the energy of a system. Evaporation is the process by which molecules undergo the a spontaneous transition from the liquid phase to the gas phase. A substance melts or freezes at a temperature called its melting point, and boils (evaporates rapidly) or. Usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or the boiling point of the substance. A phase change is a physical process in which a substance goes from one phase to another. Changes of state are examples of phase changes, or phase transitions. An interesting feature of the process of cooling the human body by evaporation is that the heat extracted by the evaporation of a gram of. Because a gas has more energy than a liquid, that means that energy must be provided to a. The melting point is the temperature at which the substance goes from a solid to a liquid (or from a. Vaporization, which is more often referred to as boiling, is the complementary process in which a chemical is converted from the liquid state of. Vaporization is the phase change that occurs when a substance converts from a liquid to a gas. In evaporation, a liquid turns into a gas; Evaporation is the opposite of condensation. All phase changes are accompanied by changes in the energy of a system. The opposite process is condensation. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID1756244 What Is A Evaporation In Phase Change Usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or the boiling point of the substance. All phase changes are accompanied by changes in the energy of a system. Vaporization is the phase change that occurs when a substance converts from a liquid to a gas. An interesting feature of the. What Is A Evaporation In Phase Change.
From www.slideserve.com
PPT Chapter 17 CHANGE OF PHASE PowerPoint Presentation, free What Is A Evaporation In Phase Change Vaporization, which is more often referred to as boiling, is the complementary process in which a chemical is converted from the liquid state of. In evaporation, a liquid turns into a gas; The opposite process is condensation. Usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or the boiling point of. What Is A Evaporation In Phase Change.
From sciencenotes.org
Phase Changes of Matter (Phase Transitions) What Is A Evaporation In Phase Change A substance melts or freezes at a temperature called its melting point, and boils (evaporates rapidly) or. Because a gas has more energy than a liquid, that means that energy must be provided to a. An interesting feature of the process of cooling the human body by evaporation is that the heat extracted by the evaporation of a gram of.. What Is A Evaporation In Phase Change.
From study.com
Phase Change Evaporation, Condensation, Freezing, Melting, Sublimation What Is A Evaporation In Phase Change Evaporation is the opposite of condensation. A substance melts or freezes at a temperature called its melting point, and boils (evaporates rapidly) or. Evaporation is the process by which molecules undergo the a spontaneous transition from the liquid phase to the gas phase. An interesting feature of the process of cooling the human body by evaporation is that the heat. What Is A Evaporation In Phase Change.
From www.geeksforgeeks.org
What is Vaporization? What Is A Evaporation In Phase Change A phase change is a physical process in which a substance goes from one phase to another. Because a gas has more energy than a liquid, that means that energy must be provided to a. Vaporization is the phase change that occurs when a substance converts from a liquid to a gas. Fusion, vaporization, and sublimation are endothermic processes, whereas. What Is A Evaporation In Phase Change.
From circuitdiagramlows.z22.web.core.windows.net
Phase Change Diagram For Water What Is A Evaporation In Phase Change A phase change is a physical process in which a substance goes from one phase to another. Changes of state are examples of phase changes, or phase transitions. A substance melts or freezes at a temperature called its melting point, and boils (evaporates rapidly) or. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes.. What Is A Evaporation In Phase Change.
From www.pinterest.com
Changes of states. Part 2 of 6. Evaporation water boiling. Phase What Is A Evaporation In Phase Change All phase changes are accompanied by changes in the energy of a system. A substance melts or freezes at a temperature called its melting point, and boils (evaporates rapidly) or. An interesting feature of the process of cooling the human body by evaporation is that the heat extracted by the evaporation of a gram of. The melting point is the. What Is A Evaporation In Phase Change.
From www.slideserve.com
PPT States of Matter Phase Change PowerPoint Presentation, free What Is A Evaporation In Phase Change The melting point is the temperature at which the substance goes from a solid to a liquid (or from a. The opposite process is condensation. Changes of state are examples of phase changes, or phase transitions. Because a gas has more energy than a liquid, that means that energy must be provided to a. In evaporation, a liquid turns into. What Is A Evaporation In Phase Change.
From stock.adobe.com
Phase change transition diagram. States matter schema. Evaporation What Is A Evaporation In Phase Change Evaporation is the opposite of condensation. A substance melts or freezes at a temperature called its melting point, and boils (evaporates rapidly) or. Usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or the boiling point of the substance. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition. What Is A Evaporation In Phase Change.
From www.researchgate.net
Phase change diagram temperature as a function of heat added. T m and What Is A Evaporation In Phase Change A phase change is a physical process in which a substance goes from one phase to another. The melting point is the temperature at which the substance goes from a solid to a liquid (or from a. Evaporation is the opposite of condensation. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. Evaporation is. What Is A Evaporation In Phase Change.
From www.britannica.com
Phase Definition & Facts Britannica What Is A Evaporation In Phase Change Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. An interesting feature of the process of cooling the human body by evaporation is that the heat extracted by the evaporation of a gram of. Changes of state are examples of phase changes, or phase transitions. Evaporation is the process by which molecules undergo the. What Is A Evaporation In Phase Change.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change What Is A Evaporation In Phase Change In evaporation, a liquid turns into a gas; Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. Evaporation is the process by which molecules undergo the a spontaneous transition from the liquid phase to the gas phase. The melting point is the temperature at which the substance goes from a solid to a liquid. What Is A Evaporation In Phase Change.
From app.emaze.com
Science Molecules on emaze What Is A Evaporation In Phase Change The opposite process is condensation. All phase changes are accompanied by changes in the energy of a system. Vaporization, which is more often referred to as boiling, is the complementary process in which a chemical is converted from the liquid state of. Usually the change occurs when adding or removing heat at a particular temperature, known as the melting point. What Is A Evaporation In Phase Change.
From www.slideserve.com
PPT States of Matter Phase Change PowerPoint Presentation, free What Is A Evaporation In Phase Change Vaporization, which is more often referred to as boiling, is the complementary process in which a chemical is converted from the liquid state of. Evaporation is the process by which molecules undergo the a spontaneous transition from the liquid phase to the gas phase. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. Because. What Is A Evaporation In Phase Change.
From www.slideserve.com
PPT Phases, Phase Changes, Chemical and Physical Changes PowerPoint What Is A Evaporation In Phase Change An interesting feature of the process of cooling the human body by evaporation is that the heat extracted by the evaporation of a gram of. The opposite process is condensation. A phase change is a physical process in which a substance goes from one phase to another. All phase changes are accompanied by changes in the energy of a system.. What Is A Evaporation In Phase Change.
From www.slideshare.net
Phase changes What Is A Evaporation In Phase Change A substance melts or freezes at a temperature called its melting point, and boils (evaporates rapidly) or. Because a gas has more energy than a liquid, that means that energy must be provided to a. Evaporation is the opposite of condensation. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. Usually the change occurs. What Is A Evaporation In Phase Change.
From stock.adobe.com
Water States of matter Phase. Change of State for Water Diagram What Is A Evaporation In Phase Change Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. A phase change is a physical process in which a substance goes from one phase to another. Usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or the boiling point of the substance. Because a gas. What Is A Evaporation In Phase Change.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID3746915 What Is A Evaporation In Phase Change A substance melts or freezes at a temperature called its melting point, and boils (evaporates rapidly) or. The melting point is the temperature at which the substance goes from a solid to a liquid (or from a. Vaporization is the phase change that occurs when a substance converts from a liquid to a gas. Evaporation is the opposite of condensation.. What Is A Evaporation In Phase Change.
From www.expii.com
Phase Change Diagrams — Overview & Examples Expii What Is A Evaporation In Phase Change Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. In evaporation, a liquid turns into a gas; Evaporation is the process by which molecules undergo the a spontaneous transition from the liquid phase to the gas phase. A substance melts or freezes at a temperature called its melting point, and boils (evaporates rapidly) or.. What Is A Evaporation In Phase Change.
From www.shmoop.com
Chemistry Phase Changes Shmoop Chemistry What Is A Evaporation In Phase Change Changes of state are examples of phase changes, or phase transitions. Evaporation is the opposite of condensation. A phase change is a physical process in which a substance goes from one phase to another. An interesting feature of the process of cooling the human body by evaporation is that the heat extracted by the evaporation of a gram of. Because. What Is A Evaporation In Phase Change.
From www.slideserve.com
PPT Chapter 23 Changes of Phase PowerPoint Presentation, free What Is A Evaporation In Phase Change A substance melts or freezes at a temperature called its melting point, and boils (evaporates rapidly) or. In evaporation, a liquid turns into a gas; Vaporization is the phase change that occurs when a substance converts from a liquid to a gas. Vaporization, which is more often referred to as boiling, is the complementary process in which a chemical is. What Is A Evaporation In Phase Change.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID5049190 What Is A Evaporation In Phase Change An interesting feature of the process of cooling the human body by evaporation is that the heat extracted by the evaporation of a gram of. The melting point is the temperature at which the substance goes from a solid to a liquid (or from a. Usually the change occurs when adding or removing heat at a particular temperature, known as. What Is A Evaporation In Phase Change.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID2437650 What Is A Evaporation In Phase Change Evaporation is the process by which molecules undergo the a spontaneous transition from the liquid phase to the gas phase. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. Because a gas has more energy than a liquid, that means that energy must be provided to a. Evaporation is the opposite of condensation. The. What Is A Evaporation In Phase Change.
From www.slideserve.com
PPT Chapter 17 CHANGE OF PHASE PowerPoint Presentation, free What Is A Evaporation In Phase Change Vaporization is the phase change that occurs when a substance converts from a liquid to a gas. Evaporation is the opposite of condensation. All phase changes are accompanied by changes in the energy of a system. Usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or the boiling point of the. What Is A Evaporation In Phase Change.
From www.slideserve.com
PPT Chapter 8 Heat Transfer & Change of Phase PowerPoint Presentation What Is A Evaporation In Phase Change Because a gas has more energy than a liquid, that means that energy must be provided to a. Usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or the boiling point of the substance. A substance melts or freezes at a temperature called its melting point, and boils (evaporates rapidly) or.. What Is A Evaporation In Phase Change.
From conceptgroupllc.com
What is phase change? Explained by Thermal Engineers What Is A Evaporation In Phase Change Vaporization is the phase change that occurs when a substance converts from a liquid to a gas. Vaporization, which is more often referred to as boiling, is the complementary process in which a chemical is converted from the liquid state of. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. Because a gas has. What Is A Evaporation In Phase Change.
From tutors.com
Evaporation Definition & Examples (Video) What Is A Evaporation In Phase Change The opposite process is condensation. Because a gas has more energy than a liquid, that means that energy must be provided to a. All phase changes are accompanied by changes in the energy of a system. Usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or the boiling point of the. What Is A Evaporation In Phase Change.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID5049190 What Is A Evaporation In Phase Change Vaporization, which is more often referred to as boiling, is the complementary process in which a chemical is converted from the liquid state of. A phase change is a physical process in which a substance goes from one phase to another. Evaporation is the process by which molecules undergo the a spontaneous transition from the liquid phase to the gas. What Is A Evaporation In Phase Change.
From www.slideserve.com
PPT Chapter 23 Changes of Phase PowerPoint Presentation, free What Is A Evaporation In Phase Change Evaporation is the process by which molecules undergo the a spontaneous transition from the liquid phase to the gas phase. Vaporization is the phase change that occurs when a substance converts from a liquid to a gas. Changes of state are examples of phase changes, or phase transitions. Vaporization, which is more often referred to as boiling, is the complementary. What Is A Evaporation In Phase Change.
From www.britannica.com
phase Definition & Facts Britannica What Is A Evaporation In Phase Change Evaporation is the opposite of condensation. A phase change is a physical process in which a substance goes from one phase to another. The melting point is the temperature at which the substance goes from a solid to a liquid (or from a. Evaporation is the process by which molecules undergo the a spontaneous transition from the liquid phase to. What Is A Evaporation In Phase Change.
From www.expii.com
Vaporization — Definition & Overview Expii What Is A Evaporation In Phase Change Because a gas has more energy than a liquid, that means that energy must be provided to a. A substance melts or freezes at a temperature called its melting point, and boils (evaporates rapidly) or. Usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or the boiling point of the substance.. What Is A Evaporation In Phase Change.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? What Is A Evaporation In Phase Change Vaporization is the phase change that occurs when a substance converts from a liquid to a gas. Because a gas has more energy than a liquid, that means that energy must be provided to a. Vaporization, which is more often referred to as boiling, is the complementary process in which a chemical is converted from the liquid state of. Usually. What Is A Evaporation In Phase Change.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID5049190 What Is A Evaporation In Phase Change Vaporization is the phase change that occurs when a substance converts from a liquid to a gas. Changes of state are examples of phase changes, or phase transitions. Usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or the boiling point of the substance. In evaporation, a liquid turns into a. What Is A Evaporation In Phase Change.
From general.chemistrysteps.com
Heat and Phase Change Diagrams Chemistry Steps What Is A Evaporation In Phase Change Vaporization, which is more often referred to as boiling, is the complementary process in which a chemical is converted from the liquid state of. All phase changes are accompanied by changes in the energy of a system. Usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or the boiling point of. What Is A Evaporation In Phase Change.
From www.slideserve.com
PPT Chapter 17 CHANGE OF PHASE PowerPoint Presentation, free What Is A Evaporation In Phase Change All phase changes are accompanied by changes in the energy of a system. Evaporation is the opposite of condensation. Evaporation is the process by which molecules undergo the a spontaneous transition from the liquid phase to the gas phase. The opposite process is condensation. Because a gas has more energy than a liquid, that means that energy must be provided. What Is A Evaporation In Phase Change.