Buffer Capacity Lab Report . In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by 1 unit. Buffer capacity is the measurement of the efficiency of the buffer solution to resist ph changes upon addition of an acid or a base. A buffer solution has the greatest capacity when the concentration ratio of the two components is 1, and better within one ph unit of pka. In the first segment of the lab, the trends for the presented buffer and strong acid, buffer and strongs base, and water and a strong base were determined. • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong bases are added. Define a buffer and explain how a buffer works.
from www.studocu.com
Define a buffer and explain how a buffer works. • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong bases are added. In the first segment of the lab, the trends for the presented buffer and strong acid, buffer and strongs base, and water and a strong base were determined. A buffer solution has the greatest capacity when the concentration ratio of the two components is 1, and better within one ph unit of pka. Buffer capacity is the measurement of the efficiency of the buffer solution to resist ph changes upon addition of an acid or a base. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by 1 unit.
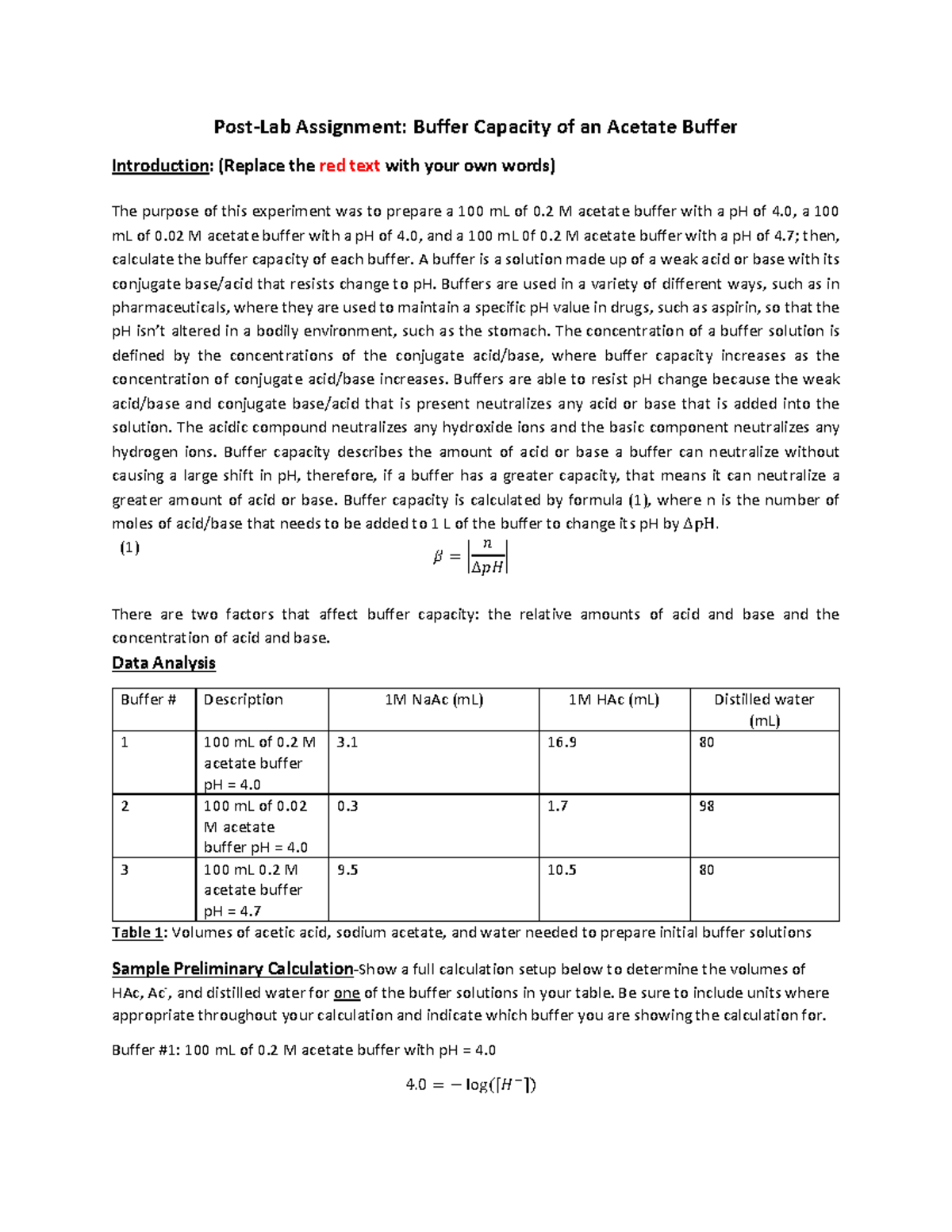
Buffer Capacity Postlab Worksheet PostLab Assignment Buffer Capacity of an Acetate Buffer
Buffer Capacity Lab Report Define a buffer and explain how a buffer works. • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong bases are added. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by 1 unit. A buffer solution has the greatest capacity when the concentration ratio of the two components is 1, and better within one ph unit of pka. Buffer capacity is the measurement of the efficiency of the buffer solution to resist ph changes upon addition of an acid or a base. Define a buffer and explain how a buffer works. In the first segment of the lab, the trends for the presented buffer and strong acid, buffer and strongs base, and water and a strong base were determined.
From www.chegg.com
Solved BUFFERS BUFFERS AND BUFFER CAPACITY . INTRODUCTION Buffer Capacity Lab Report Buffer capacity is the measurement of the efficiency of the buffer solution to resist ph changes upon addition of an acid or a base. In the first segment of the lab, the trends for the presented buffer and strong acid, buffer and strongs base, and water and a strong base were determined. • investigate the nature of buffer solutions with. Buffer Capacity Lab Report.
From www.studocu.com
Chem 2360 lab report 2 Lab Report II Experiment 2 Buffers, Buffer Capacity and Amino Acid Buffer Capacity Lab Report In the first segment of the lab, the trends for the presented buffer and strong acid, buffer and strongs base, and water and a strong base were determined. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its. Buffer Capacity Lab Report.
From www.studocu.com
More About Buffers Report Part 1 Buffer Capacity and Buffer RangeInvestigated Using a H 2 Buffer Capacity Lab Report • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong bases are added. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by. Buffer Capacity Lab Report.
From www.studypool.com
SOLUTION Ph and buffer system Lab Report Studypool Buffer Capacity Lab Report In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by 1 unit. Buffer capacity is the measurement of the efficiency of the buffer solution to resist ph changes upon addition of an acid or. Buffer Capacity Lab Report.
From study.com
Identifying Buffer Capacity Chemistry Buffer Capacity Lab Report In the first segment of the lab, the trends for the presented buffer and strong acid, buffer and strongs base, and water and a strong base were determined. A buffer solution has the greatest capacity when the concentration ratio of the two components is 1, and better within one ph unit of pka. Define a buffer and explain how a. Buffer Capacity Lab Report.
From www.studocu.com
Practical 3 Buffer Preparation and Buffering Capacity Determination PRACTICAL 2 PREPARATION OF Buffer Capacity Lab Report In the first segment of the lab, the trends for the presented buffer and strong acid, buffer and strongs base, and water and a strong base were determined. • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong bases are added. Buffer capacity is the measurement of the efficiency. Buffer Capacity Lab Report.
From www.chegg.com
FERS. BUFFERS AND BUFFER CAPACITY ODUCTION LABORATORY Buffer Capacity Lab Report In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by 1 unit. Buffer capacity is the measurement of the efficiency of the buffer solution to resist ph changes upon addition of an acid or. Buffer Capacity Lab Report.
From www.chegg.com
Solved PreLab Investigation 11 How does the buffer Buffer Capacity Lab Report A buffer solution has the greatest capacity when the concentration ratio of the two components is 1, and better within one ph unit of pka. Define a buffer and explain how a buffer works. Buffer capacity is the measurement of the efficiency of the buffer solution to resist ph changes upon addition of an acid or a base. In the. Buffer Capacity Lab Report.
From www.chegg.com
Solved Buffer Capacity Lab Report Define a buffer and explain how a buffer works. A buffer solution has the greatest capacity when the concentration ratio of the two components is 1, and better within one ph unit of pka. Buffer capacity is the measurement of the efficiency of the buffer solution to resist ph changes upon addition of an acid or a base. • investigate. Buffer Capacity Lab Report.
From studylib.net
Buffer Lab Buffer Capacity Lab Report Define a buffer and explain how a buffer works. • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong bases are added. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of. Buffer Capacity Lab Report.
From www.studocu.com
Lab report 2 CHEM271 EXPERIMENT 2 BUFFERS AND BIOCHEMICAL ANALYSIS PART 1 Buffer Preparation Buffer Capacity Lab Report Buffer capacity is the measurement of the efficiency of the buffer solution to resist ph changes upon addition of an acid or a base. • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong bases are added. Define a buffer and explain how a buffer works. A buffer solution. Buffer Capacity Lab Report.
From www.slideserve.com
PPT Buffer Capacity Lab PowerPoint Presentation, free download ID2813844 Buffer Capacity Lab Report Buffer capacity is the measurement of the efficiency of the buffer solution to resist ph changes upon addition of an acid or a base. • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong bases are added. Define a buffer and explain how a buffer works. A buffer solution. Buffer Capacity Lab Report.
From www.studocu.com
Lab 2 The scientific Method Buffer Lab 2 Buffers. Worth = (points earned/18)*15 = total Buffer Capacity Lab Report Buffer capacity is the measurement of the efficiency of the buffer solution to resist ph changes upon addition of an acid or a base. Define a buffer and explain how a buffer works. • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong bases are added. A buffer solution. Buffer Capacity Lab Report.
From www.coursehero.com
[Solved] Buffer Capacity Lab Report A buffer solution has the greatest capacity when the concentration ratio of the two components is 1, and better within one ph unit of pka. • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong bases are added. Buffer capacity is the measurement of the efficiency of the buffer. Buffer Capacity Lab Report.
From studylib.net
Preparing Buffers and Buffer Capacity Buffer Capacity Lab Report • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong bases are added. Buffer capacity is the measurement of the efficiency of the buffer solution to resist ph changes upon addition of an acid or a base. In more rigorous terms, buffer capacity is defined as the number of. Buffer Capacity Lab Report.
From www.studocu.com
Buffer Capacity Postlab Worksheet PostLab Assignment Buffer Capacity of an Acetate Buffer Buffer Capacity Lab Report • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong bases are added. Buffer capacity is the measurement of the efficiency of the buffer solution to resist ph changes upon addition of an acid or a base. A buffer solution has the greatest capacity when the concentration ratio of. Buffer Capacity Lab Report.
From www.scribd.com
Buffer Lab Report PDF Buffer Solution Acid Buffer Capacity Lab Report A buffer solution has the greatest capacity when the concentration ratio of the two components is 1, and better within one ph unit of pka. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change. Buffer Capacity Lab Report.
From chemexpert.net
Mastering Buffer Capacity Your Guide To Maintaining Chemical Harmony Buffer Capacity Lab Report A buffer solution has the greatest capacity when the concentration ratio of the two components is 1, and better within one ph unit of pka. Define a buffer and explain how a buffer works. In the first segment of the lab, the trends for the presented buffer and strong acid, buffer and strongs base, and water and a strong base. Buffer Capacity Lab Report.
From www.slideserve.com
PPT Buffer Capacity Lab PowerPoint Presentation, free download ID2813844 Buffer Capacity Lab Report A buffer solution has the greatest capacity when the concentration ratio of the two components is 1, and better within one ph unit of pka. Define a buffer and explain how a buffer works. In the first segment of the lab, the trends for the presented buffer and strong acid, buffer and strongs base, and water and a strong base. Buffer Capacity Lab Report.
From www.slideserve.com
PPT Determination of Buffer Capacity PowerPoint Presentation, free download ID1994739 Buffer Capacity Lab Report A buffer solution has the greatest capacity when the concentration ratio of the two components is 1, and better within one ph unit of pka. Define a buffer and explain how a buffer works. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter. Buffer Capacity Lab Report.
From www.numerade.com
SOLVED You will analyze the data for both buffers using the data from your "virtual" lab Buffer Capacity Lab Report • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong bases are added. In the first segment of the lab, the trends for the presented buffer and strong acid, buffer and strongs base, and water and a strong base were determined. Buffer capacity is the measurement of the efficiency. Buffer Capacity Lab Report.
From www.coursehero.com
[Solved] Buffer Capacity Lab Report In the first segment of the lab, the trends for the presented buffer and strong acid, buffer and strongs base, and water and a strong base were determined. • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong bases are added. Define a buffer and explain how a buffer. Buffer Capacity Lab Report.
From www.studocu.com
Buffers and Buffer Capacity Chem 1252L4/9/ Buffers and Buffer Capacity Introduction Buffer Buffer Capacity Lab Report Define a buffer and explain how a buffer works. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by 1 unit. A buffer solution has the greatest capacity when the concentration ratio of the. Buffer Capacity Lab Report.
From www.slideserve.com
PPT Buffer Capacity Lab PowerPoint Presentation, free download ID2813844 Buffer Capacity Lab Report • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong bases are added. In the first segment of the lab, the trends for the presented buffer and strong acid, buffer and strongs base, and water and a strong base were determined. Define a buffer and explain how a buffer. Buffer Capacity Lab Report.
From www.slideserve.com
PPT Determination of Buffer Capacity PowerPoint Presentation, free download ID5759485 Buffer Capacity Lab Report Define a buffer and explain how a buffer works. Buffer capacity is the measurement of the efficiency of the buffer solution to resist ph changes upon addition of an acid or a base. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of. Buffer Capacity Lab Report.
From www.scribd.com
Laboratory Worksheet 2 Buffers and Buffer Capacity PDF Buffer Solution Ph Buffer Capacity Lab Report • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong bases are added. Define a buffer and explain how a buffer works. Buffer capacity is the measurement of the efficiency of the buffer solution to resist ph changes upon addition of an acid or a base. A buffer solution. Buffer Capacity Lab Report.
From studylib.net
Buffer Capacity Buffer Capacity Lab Report In the first segment of the lab, the trends for the presented buffer and strong acid, buffer and strongs base, and water and a strong base were determined. A buffer solution has the greatest capacity when the concentration ratio of the two components is 1, and better within one ph unit of pka. In more rigorous terms, buffer capacity is. Buffer Capacity Lab Report.
From www.slideserve.com
PPT Determination of Buffer Capacity PowerPoint Presentation, free download ID1994739 Buffer Capacity Lab Report In the first segment of the lab, the trends for the presented buffer and strong acid, buffer and strongs base, and water and a strong base were determined. Buffer capacity is the measurement of the efficiency of the buffer solution to resist ph changes upon addition of an acid or a base. • investigate the nature of buffer solutions with. Buffer Capacity Lab Report.
From www.vrogue.co
Lab 8 How Do Buffers Work Lab 9 How Do Buffers Work T vrogue.co Buffer Capacity Lab Report • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong bases are added. In the first segment of the lab, the trends for the presented buffer and strong acid, buffer and strongs base, and water and a strong base were determined. Buffer capacity is the measurement of the efficiency. Buffer Capacity Lab Report.
From www.slideserve.com
PPT Buffer Capacity Lab PowerPoint Presentation, free download ID2813844 Buffer Capacity Lab Report In the first segment of the lab, the trends for the presented buffer and strong acid, buffer and strongs base, and water and a strong base were determined. A buffer solution has the greatest capacity when the concentration ratio of the two components is 1, and better within one ph unit of pka. • investigate the nature of buffer solutions. Buffer Capacity Lab Report.
From pdfprof.com
determining buffer capacity experiment Buffer Capacity Lab Report Define a buffer and explain how a buffer works. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by 1 unit. A buffer solution has the greatest capacity when the concentration ratio of the. Buffer Capacity Lab Report.
From www.chegg.com
full lab report on buffering capacity of alkaseltzer Buffer Capacity Lab Report Define a buffer and explain how a buffer works. Buffer capacity is the measurement of the efficiency of the buffer solution to resist ph changes upon addition of an acid or a base. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of. Buffer Capacity Lab Report.
From www.studypool.com
SOLUTION Ph and buffer system Lab Report Studypool Buffer Capacity Lab Report Buffer capacity is the measurement of the efficiency of the buffer solution to resist ph changes upon addition of an acid or a base. A buffer solution has the greatest capacity when the concentration ratio of the two components is 1, and better within one ph unit of pka. In the first segment of the lab, the trends for the. Buffer Capacity Lab Report.
From www.studypool.com
SOLUTION 4 buffering capacity Studypool Buffer Capacity Lab Report • investigate the nature of buffer solutions with respect to ph, buffer region, and buffer capacity when strong acids and strong bases are added. In more rigorous terms, buffer capacity is defined as the number of moles of an acid or base that has to be added to 1 liter of a buffer to cause its ph to change by. Buffer Capacity Lab Report.
From www.studypool.com
SOLUTION Biochemistry laboratory final report buffer system acid base reaction Studypool Buffer Capacity Lab Report Buffer capacity is the measurement of the efficiency of the buffer solution to resist ph changes upon addition of an acid or a base. In the first segment of the lab, the trends for the presented buffer and strong acid, buffer and strongs base, and water and a strong base were determined. In more rigorous terms, buffer capacity is defined. Buffer Capacity Lab Report.