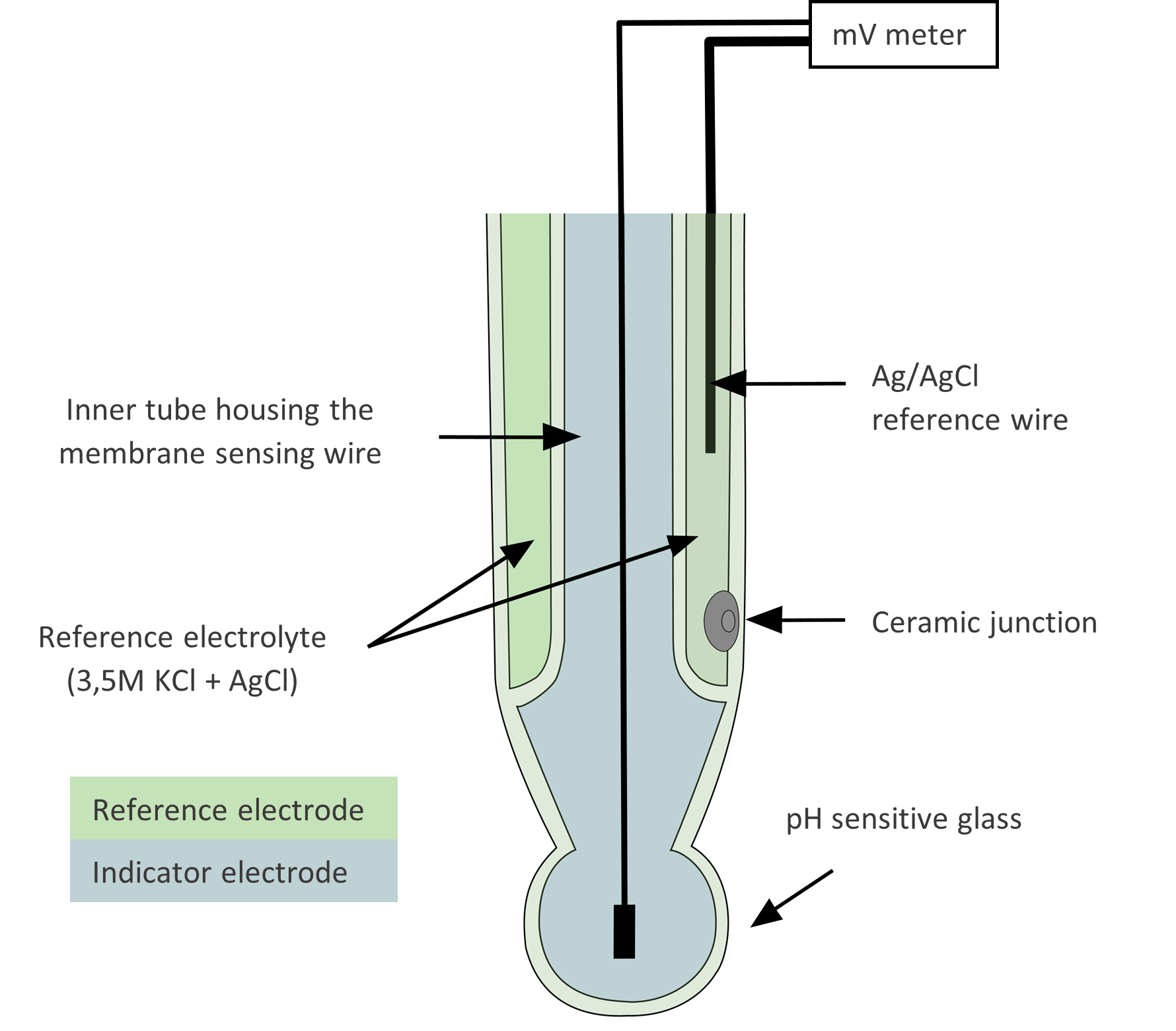
What Is A Reference Electrode Used For . In a reversible electrode a small cathodic current produces the. It serves as a stable. Values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard hydrogen electrode is used as a reference. Reference electrodes are an essential component of electrochemical measurement, characterized by its stable and known reference potential. The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a. Reference electrodes used are preferably reversible type electrodes. This is accomplished by placing a second electrode, called the reference electrode, in the cell and measuring potential as the energy difference between the two electrodes. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements.
from blog.hannaservice.eu
Values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard hydrogen electrode is used as a reference. In a reversible electrode a small cathodic current produces the. It serves as a stable. Reference electrodes used are preferably reversible type electrodes. The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a. This is accomplished by placing a second electrode, called the reference electrode, in the cell and measuring potential as the energy difference between the two electrodes. Reference electrodes are an essential component of electrochemical measurement, characterized by its stable and known reference potential. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements.
Choose the right Electrode for your Samples Hanna Instruments Africa
What Is A Reference Electrode Used For In a reversible electrode a small cathodic current produces the. This is accomplished by placing a second electrode, called the reference electrode, in the cell and measuring potential as the energy difference between the two electrodes. It serves as a stable. In a reversible electrode a small cathodic current produces the. Reference electrodes used are preferably reversible type electrodes. Values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard hydrogen electrode is used as a reference. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. Reference electrodes are an essential component of electrochemical measurement, characterized by its stable and known reference potential. The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a.
From www.hamiltoncompany.com
The pH Reference Electrode Process Analytics Hamilton Company What Is A Reference Electrode Used For Reference electrodes are an essential component of electrochemical measurement, characterized by its stable and known reference potential. Values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard hydrogen electrode is used as a reference. A reference electrode is a stable and known electrode potential used as a comparison point in. What Is A Reference Electrode Used For.
From blog.hannaservice.eu
Choose the right Electrode for your Samples Hanna Instruments Africa What Is A Reference Electrode Used For The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a. In a reversible electrode a small cathodic current produces the. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. This is accomplished by placing a second electrode, called the. What Is A Reference Electrode Used For.
From www.researchgate.net
a Reference electrode Cu/CuSO4 1 copper electrode, 2 plug,... Download Scientific Diagram What Is A Reference Electrode Used For It serves as a stable. In a reversible electrode a small cathodic current produces the. Reference electrodes are an essential component of electrochemical measurement, characterized by its stable and known reference potential. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. Values of e 0 are most often reported as the. What Is A Reference Electrode Used For.
From www.researchgate.net
Working electrode. Reference Electrode The reference electrode used... Download Scientific Diagram What Is A Reference Electrode Used For Values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard hydrogen electrode is used as a reference. Reference electrodes used are preferably reversible type electrodes. It serves as a stable. The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference. What Is A Reference Electrode Used For.
From askfilo.com
The electrode potential of calomel electrode used as a reference electrod.. What Is A Reference Electrode Used For Reference electrodes are an essential component of electrochemical measurement, characterized by its stable and known reference potential. Reference electrodes used are preferably reversible type electrodes. The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a. In a reversible electrode a small cathodic current produces the. A reference electrode. What Is A Reference Electrode Used For.
From www.ecscell.com
Reference electrode with solid polymer electrolyte Ecscell What Is A Reference Electrode Used For A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. Values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard hydrogen electrode is used as a reference. Reference electrodes used are preferably reversible type electrodes. It serves as a stable. This is. What Is A Reference Electrode Used For.
From www.youtube.com
Saturated calomel electrode (SCE)/ Secondary reference electrode YouTube What Is A Reference Electrode Used For This is accomplished by placing a second electrode, called the reference electrode, in the cell and measuring potential as the energy difference between the two electrodes. Reference electrodes used are preferably reversible type electrodes. Values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard hydrogen electrode is used as a. What Is A Reference Electrode Used For.
From gaskatel.de
Reference electrode Gaskatel What Is A Reference Electrode Used For Reference electrodes are an essential component of electrochemical measurement, characterized by its stable and known reference potential. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. This is accomplished by placing a second electrode, called the reference electrode, in the cell and measuring potential as the energy difference between the two. What Is A Reference Electrode Used For.
From www.researchgate.net
Schematic description for reference electrode connection to MFC Download Scientific Diagram What Is A Reference Electrode Used For A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. Reference electrodes used are preferably reversible type electrodes. In a reversible electrode a small cathodic current produces the. This is accomplished by placing a second electrode, called the reference electrode, in the cell and measuring potential as the energy difference between the. What Is A Reference Electrode Used For.
From www.youtube.com
Reference Electrodes YouTube What Is A Reference Electrode Used For This is accomplished by placing a second electrode, called the reference electrode, in the cell and measuring potential as the energy difference between the two electrodes. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. Reference electrodes used are preferably reversible type electrodes. It serves as a stable. In a reversible. What Is A Reference Electrode Used For.
From semcouniversity.com
How the three electrode system works Semco University Semco university All about the What Is A Reference Electrode Used For The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a. Values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard hydrogen electrode is used as a reference. A reference electrode is a stable and known electrode potential used. What Is A Reference Electrode Used For.
From mungfali.com
Reference Electrode Diagram What Is A Reference Electrode Used For Reference electrodes are an essential component of electrochemical measurement, characterized by its stable and known reference potential. It serves as a stable. This is accomplished by placing a second electrode, called the reference electrode, in the cell and measuring potential as the energy difference between the two electrodes. Values of e 0 are most often reported as the potential measured. What Is A Reference Electrode Used For.
From www.gamry.com
Reference Electrodes Saturated CalomelSilver ChlorideMercury Oxide What Is A Reference Electrode Used For Reference electrodes used are preferably reversible type electrodes. It serves as a stable. This is accomplished by placing a second electrode, called the reference electrode, in the cell and measuring potential as the energy difference between the two electrodes. In a reversible electrode a small cathodic current produces the. Values of e 0 are most often reported as the potential. What Is A Reference Electrode Used For.
From armingroup.com
Reference electrodes What Is A Reference Electrode Used For Reference electrodes used are preferably reversible type electrodes. In a reversible electrode a small cathodic current produces the. Reference electrodes are an essential component of electrochemical measurement, characterized by its stable and known reference potential. This is accomplished by placing a second electrode, called the reference electrode, in the cell and measuring potential as the energy difference between the two. What Is A Reference Electrode Used For.
From www.scribd.com
Reference Electrode What Is A Reference Electrode Used For In a reversible electrode a small cathodic current produces the. The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a. Reference electrodes used are preferably reversible type electrodes. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. Reference electrodes. What Is A Reference Electrode Used For.
From www.researchgate.net
Design of the copper/copper sulfate reference electrode for nonsight... Download Scientific What Is A Reference Electrode Used For Values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard hydrogen electrode is used as a reference. Reference electrodes are an essential component of electrochemical measurement, characterized by its stable and known reference potential. A reference electrode is a stable and known electrode potential used as a comparison point in. What Is A Reference Electrode Used For.
From mungfali.com
Reference Electrode Diagram What Is A Reference Electrode Used For Reference electrodes used are preferably reversible type electrodes. This is accomplished by placing a second electrode, called the reference electrode, in the cell and measuring potential as the energy difference between the two electrodes. Reference electrodes are an essential component of electrochemical measurement, characterized by its stable and known reference potential. Values of e 0 are most often reported as. What Is A Reference Electrode Used For.
From www.researchgate.net
(PDF) Reference Electrodes What Is A Reference Electrode Used For Values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard hydrogen electrode is used as a reference. In a reversible electrode a small cathodic current produces the. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. Reference electrodes are an essential. What Is A Reference Electrode Used For.
From studylib.net
Reference Electrodes What Is A Reference Electrode Used For It serves as a stable. Values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard hydrogen electrode is used as a reference. The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a. In a reversible electrode a small. What Is A Reference Electrode Used For.
From analyteguru.com
How do I know which reference electrode to use? What Is A Reference Electrode Used For Reference electrodes are an essential component of electrochemical measurement, characterized by its stable and known reference potential. It serves as a stable. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. Values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard. What Is A Reference Electrode Used For.
From www.youtube.com
Standard Hydrogen Electrode (SHE)/ Primary reference electrode Gas electrode YouTube What Is A Reference Electrode Used For Reference electrodes are an essential component of electrochemical measurement, characterized by its stable and known reference potential. This is accomplished by placing a second electrode, called the reference electrode, in the cell and measuring potential as the energy difference between the two electrodes. Values of e 0 are most often reported as the potential measured in an electrochemical cell for. What Is A Reference Electrode Used For.
From www.snexplores.org
Explainer What is an electrode? What Is A Reference Electrode Used For In a reversible electrode a small cathodic current produces the. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. Values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard hydrogen electrode is used as a reference. It serves as a stable.. What Is A Reference Electrode Used For.
From read.cholonautas.edu.pe
What Is Reference Electrode With Example Printable Templates Free What Is A Reference Electrode Used For This is accomplished by placing a second electrode, called the reference electrode, in the cell and measuring potential as the energy difference between the two electrodes. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. It serves as a stable. The potential of one electrode—the working or indicator electrode—responds to the. What Is A Reference Electrode Used For.
From www.slideserve.com
PPT Uses of Reference Electrodes for various purposes PowerPoint Presentation ID11897230 What Is A Reference Electrode Used For The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a. This is accomplished by placing a second electrode, called the reference electrode, in the cell and measuring potential as the energy difference between the two electrodes. It serves as a stable. Reference electrodes used are preferably reversible type. What Is A Reference Electrode Used For.
From byjus.com
79.What is reference electrode What Is A Reference Electrode Used For Reference electrodes are an essential component of electrochemical measurement, characterized by its stable and known reference potential. Values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard hydrogen electrode is used as a reference. A reference electrode is a stable and known electrode potential used as a comparison point in. What Is A Reference Electrode Used For.
From www.aliexpress.com
MercurymercuryoxidereferenceelectrodeHgHgO.jpg What Is A Reference Electrode Used For This is accomplished by placing a second electrode, called the reference electrode, in the cell and measuring potential as the energy difference between the two electrodes. The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a. Reference electrodes are an essential component of electrochemical measurement, characterized by its. What Is A Reference Electrode Used For.
From www.mdpi.com
Encyclopedia Free FullText Reference Electrodes What Is A Reference Electrode Used For The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. Reference electrodes are an essential component of electrochemical measurement, characterized by its stable and known reference potential. It serves as. What Is A Reference Electrode Used For.
From alevelchemistry.co.uk
Electrodes Facts, Summary & Definition Chemistry Revision What Is A Reference Electrode Used For It serves as a stable. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. Values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard hydrogen electrode is used as a reference. Reference electrodes used are preferably reversible type electrodes. Reference electrodes. What Is A Reference Electrode Used For.
From www.gamry.com
Reference Electrodes Saturated CalomelSilver ChlorideMercury Oxide What Is A Reference Electrode Used For This is accomplished by placing a second electrode, called the reference electrode, in the cell and measuring potential as the energy difference between the two electrodes. Reference electrodes are an essential component of electrochemical measurement, characterized by its stable and known reference potential. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical. What Is A Reference Electrode Used For.
From www.pipe-st.com
Reference electrodesPipeline systems and technologies What Is A Reference Electrode Used For A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. In a reversible electrode a small cathodic current produces the. Reference electrodes used are preferably reversible type electrodes. This is accomplished by placing a second electrode, called the reference electrode, in the cell and measuring potential as the energy difference between the. What Is A Reference Electrode Used For.
From www.researchgate.net
"Open" and "close" designs of the reference electrode compartment of... Download Scientific What Is A Reference Electrode Used For Reference electrodes used are preferably reversible type electrodes. This is accomplished by placing a second electrode, called the reference electrode, in the cell and measuring potential as the energy difference between the two electrodes. A reference electrode is a stable and known electrode potential used as a comparison point in electrochemical measurements. In a reversible electrode a small cathodic current. What Is A Reference Electrode Used For.
From stock.adobe.com
A Standard Hydrogen Electrode (SHE) is an electrode that scientists use as a reference electrode What Is A Reference Electrode Used For Values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard hydrogen electrode is used as a reference. Reference electrodes used are preferably reversible type electrodes. In a reversible electrode a small cathodic current produces the. The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the. What Is A Reference Electrode Used For.
From mungfali.com
Reference Electrode Diagram What Is A Reference Electrode Used For It serves as a stable. The potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a. Values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard hydrogen electrode is used as a reference. Reference electrodes used are preferably reversible. What Is A Reference Electrode Used For.
From mungfali.com
Types Of Electrodes What Is A Reference Electrode Used For Reference electrodes used are preferably reversible type electrodes. Values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard hydrogen electrode is used as a reference. This is accomplished by placing a second electrode, called the reference electrode, in the cell and measuring potential as the energy difference between the two. What Is A Reference Electrode Used For.
From www.researchgate.net
A. Placement of Reference Electrode. Download Scientific Diagram What Is A Reference Electrode Used For Values of e 0 are most often reported as the potential measured in an electrochemical cell for which the standard hydrogen electrode is used as a reference. Reference electrodes are an essential component of electrochemical measurement, characterized by its stable and known reference potential. It serves as a stable. A reference electrode is a stable and known electrode potential used. What Is A Reference Electrode Used For.