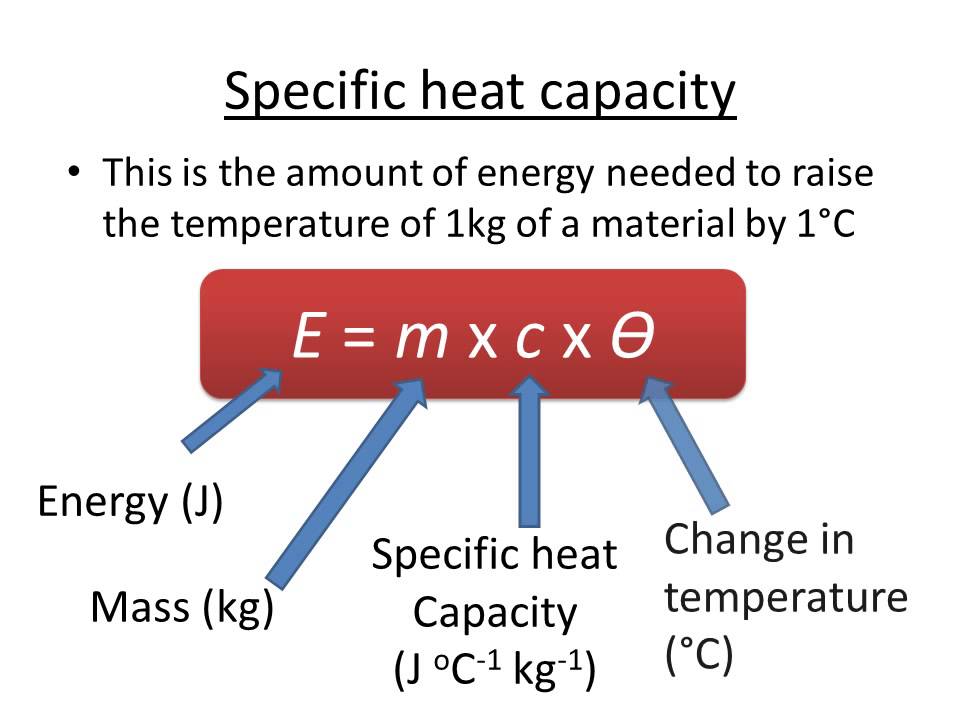
What Is The Highest Heat Capacity . Specific heat is defined as the amount of heat one gram of a substance must absorb or. Heat capacity is defined as the amount of heat energy required to raise the temperature of a given quantity of matter by one degree celsius. Heat capacity is the amount of heat needed to raise the temperature of an object by one unit. 55 rows find the volumetric, mass and molar heat capacities of various substances and materials, including air, at different. Heat capacity for a given matter depends on its size or. Find out the specific heat values of common substances and how water's. Water has the highest specific heat capacity of any liquid. The si unit of heat capacity is joule per kelvin (j/k),. Learn what specific heat and heat capacity are, how they are calculated, and what units they use. Find the specific heat of various products, such as metals, liquids, solids, gases, and organic and inorganic substances.
from getrevising.co.uk
55 rows find the volumetric, mass and molar heat capacities of various substances and materials, including air, at different. Find out the specific heat values of common substances and how water's. Find the specific heat of various products, such as metals, liquids, solids, gases, and organic and inorganic substances. The si unit of heat capacity is joule per kelvin (j/k),. Learn what specific heat and heat capacity are, how they are calculated, and what units they use. Heat capacity is the amount of heat needed to raise the temperature of an object by one unit. Heat capacity for a given matter depends on its size or. Water has the highest specific heat capacity of any liquid. Specific heat is defined as the amount of heat one gram of a substance must absorb or. Heat capacity is defined as the amount of heat energy required to raise the temperature of a given quantity of matter by one degree celsius.
Physicsend of year 9 Revision Cards in GCSE Physics
What Is The Highest Heat Capacity Heat capacity is defined as the amount of heat energy required to raise the temperature of a given quantity of matter by one degree celsius. Learn what specific heat and heat capacity are, how they are calculated, and what units they use. Heat capacity is defined as the amount of heat energy required to raise the temperature of a given quantity of matter by one degree celsius. 55 rows find the volumetric, mass and molar heat capacities of various substances and materials, including air, at different. Heat capacity for a given matter depends on its size or. Find the specific heat of various products, such as metals, liquids, solids, gases, and organic and inorganic substances. Specific heat is defined as the amount of heat one gram of a substance must absorb or. Find out the specific heat values of common substances and how water's. The si unit of heat capacity is joule per kelvin (j/k),. Heat capacity is the amount of heat needed to raise the temperature of an object by one unit. Water has the highest specific heat capacity of any liquid.
From www.slideshare.net
Heat Capacity What Is The Highest Heat Capacity Learn what specific heat and heat capacity are, how they are calculated, and what units they use. Heat capacity is defined as the amount of heat energy required to raise the temperature of a given quantity of matter by one degree celsius. The si unit of heat capacity is joule per kelvin (j/k),. Find the specific heat of various products,. What Is The Highest Heat Capacity.
From getrevising.co.uk
Physicsend of year 9 Revision Cards in GCSE Physics What Is The Highest Heat Capacity Heat capacity is the amount of heat needed to raise the temperature of an object by one unit. Heat capacity is defined as the amount of heat energy required to raise the temperature of a given quantity of matter by one degree celsius. Find the specific heat of various products, such as metals, liquids, solids, gases, and organic and inorganic. What Is The Highest Heat Capacity.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Is The Highest Heat Capacity Find the specific heat of various products, such as metals, liquids, solids, gases, and organic and inorganic substances. Learn what specific heat and heat capacity are, how they are calculated, and what units they use. The si unit of heat capacity is joule per kelvin (j/k),. Find out the specific heat values of common substances and how water's. Heat capacity. What Is The Highest Heat Capacity.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Is The Highest Heat Capacity 55 rows find the volumetric, mass and molar heat capacities of various substances and materials, including air, at different. Find out the specific heat values of common substances and how water's. Water has the highest specific heat capacity of any liquid. Heat capacity is defined as the amount of heat energy required to raise the temperature of a given quantity. What Is The Highest Heat Capacity.
From www.slideserve.com
PPT Thermal Properties PowerPoint Presentation, free download ID What Is The Highest Heat Capacity Specific heat is defined as the amount of heat one gram of a substance must absorb or. Find the specific heat of various products, such as metals, liquids, solids, gases, and organic and inorganic substances. Heat capacity is defined as the amount of heat energy required to raise the temperature of a given quantity of matter by one degree celsius.. What Is The Highest Heat Capacity.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube What Is The Highest Heat Capacity Water has the highest specific heat capacity of any liquid. Specific heat is defined as the amount of heat one gram of a substance must absorb or. 55 rows find the volumetric, mass and molar heat capacities of various substances and materials, including air, at different. Find out the specific heat values of common substances and how water's. The si. What Is The Highest Heat Capacity.
From www.youtube.com
What Is The Difference Between Specific Heat Capacity, Heat Capacity What Is The Highest Heat Capacity Find out the specific heat values of common substances and how water's. Heat capacity for a given matter depends on its size or. 55 rows find the volumetric, mass and molar heat capacities of various substances and materials, including air, at different. Specific heat is defined as the amount of heat one gram of a substance must absorb or. Heat. What Is The Highest Heat Capacity.
From dxoqtwvpm.blob.core.windows.net
What Is Heat Capacity In Materials at James Eng blog What Is The Highest Heat Capacity Water has the highest specific heat capacity of any liquid. The si unit of heat capacity is joule per kelvin (j/k),. Heat capacity is defined as the amount of heat energy required to raise the temperature of a given quantity of matter by one degree celsius. Find out the specific heat values of common substances and how water's. Specific heat. What Is The Highest Heat Capacity.
From www.slideserve.com
PPT CHAPTER 19 THERMAL PROPERTIES PowerPoint Presentation, free What Is The Highest Heat Capacity The si unit of heat capacity is joule per kelvin (j/k),. Find the specific heat of various products, such as metals, liquids, solids, gases, and organic and inorganic substances. Find out the specific heat values of common substances and how water's. Specific heat is defined as the amount of heat one gram of a substance must absorb or. Heat capacity. What Is The Highest Heat Capacity.
From www.slideshare.net
Heat Capacity What Is The Highest Heat Capacity Specific heat is defined as the amount of heat one gram of a substance must absorb or. Heat capacity is the amount of heat needed to raise the temperature of an object by one unit. 55 rows find the volumetric, mass and molar heat capacities of various substances and materials, including air, at different. Water has the highest specific heat. What Is The Highest Heat Capacity.
From www.careerpower.in
What is Heat Capacity Definition, Equations, Examples and Types What Is The Highest Heat Capacity Water has the highest specific heat capacity of any liquid. Specific heat is defined as the amount of heat one gram of a substance must absorb or. Learn what specific heat and heat capacity are, how they are calculated, and what units they use. Find out the specific heat values of common substances and how water's. Heat capacity is the. What Is The Highest Heat Capacity.
From www.slideserve.com
PPT The Chemistry of Life PowerPoint Presentation, free download ID What Is The Highest Heat Capacity Water has the highest specific heat capacity of any liquid. Find the specific heat of various products, such as metals, liquids, solids, gases, and organic and inorganic substances. Learn what specific heat and heat capacity are, how they are calculated, and what units they use. Specific heat is defined as the amount of heat one gram of a substance must. What Is The Highest Heat Capacity.
From www.studypool.com
SOLUTION Specific heat capacity definition, importance, and formula What Is The Highest Heat Capacity 55 rows find the volumetric, mass and molar heat capacities of various substances and materials, including air, at different. Water has the highest specific heat capacity of any liquid. Specific heat is defined as the amount of heat one gram of a substance must absorb or. Find the specific heat of various products, such as metals, liquids, solids, gases, and. What Is The Highest Heat Capacity.
From studylib.net
Heat Capacity of Metals PreLab What Is The Highest Heat Capacity Learn what specific heat and heat capacity are, how they are calculated, and what units they use. Find out the specific heat values of common substances and how water's. Find the specific heat of various products, such as metals, liquids, solids, gases, and organic and inorganic substances. The si unit of heat capacity is joule per kelvin (j/k),. 55 rows. What Is The Highest Heat Capacity.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Is The Highest Heat Capacity Find out the specific heat values of common substances and how water's. Specific heat is defined as the amount of heat one gram of a substance must absorb or. 55 rows find the volumetric, mass and molar heat capacities of various substances and materials, including air, at different. Heat capacity for a given matter depends on its size or. Learn. What Is The Highest Heat Capacity.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download What Is The Highest Heat Capacity Specific heat is defined as the amount of heat one gram of a substance must absorb or. 55 rows find the volumetric, mass and molar heat capacities of various substances and materials, including air, at different. Heat capacity is the amount of heat needed to raise the temperature of an object by one unit. The si unit of heat capacity. What Is The Highest Heat Capacity.
From www.slideserve.com
PPT Chapter10 Temperature and Heat PowerPoint Presentation, free What Is The Highest Heat Capacity The si unit of heat capacity is joule per kelvin (j/k),. Heat capacity is the amount of heat needed to raise the temperature of an object by one unit. Heat capacity for a given matter depends on its size or. 55 rows find the volumetric, mass and molar heat capacities of various substances and materials, including air, at different. Find. What Is The Highest Heat Capacity.
From www.careerpower.in
What is Heat Capacity Definition, Equations, Examples and Types What Is The Highest Heat Capacity Heat capacity is the amount of heat needed to raise the temperature of an object by one unit. Find out the specific heat values of common substances and how water's. Water has the highest specific heat capacity of any liquid. Specific heat is defined as the amount of heat one gram of a substance must absorb or. Heat capacity for. What Is The Highest Heat Capacity.
From www.wikihow.com
How to Calculate Heat Capacity 8 Steps (with Pictures) wikiHow What Is The Highest Heat Capacity Heat capacity is defined as the amount of heat energy required to raise the temperature of a given quantity of matter by one degree celsius. Find the specific heat of various products, such as metals, liquids, solids, gases, and organic and inorganic substances. The si unit of heat capacity is joule per kelvin (j/k),. Find out the specific heat values. What Is The Highest Heat Capacity.
From klaobjgle.blob.core.windows.net
What Is Specific Heat Capacity Geography at Daniel Whittington blog What Is The Highest Heat Capacity Find out the specific heat values of common substances and how water's. Specific heat is defined as the amount of heat one gram of a substance must absorb or. Heat capacity is defined as the amount of heat energy required to raise the temperature of a given quantity of matter by one degree celsius. 55 rows find the volumetric, mass. What Is The Highest Heat Capacity.
From serc.carleton.edu
Heat Capacity What Is The Highest Heat Capacity Find out the specific heat values of common substances and how water's. Heat capacity for a given matter depends on its size or. The si unit of heat capacity is joule per kelvin (j/k),. Heat capacity is defined as the amount of heat energy required to raise the temperature of a given quantity of matter by one degree celsius. Find. What Is The Highest Heat Capacity.
From www.wikihow.com
How to Calculate Heat Capacity 8 Steps (with Pictures) wikiHow What Is The Highest Heat Capacity Water has the highest specific heat capacity of any liquid. Find the specific heat of various products, such as metals, liquids, solids, gases, and organic and inorganic substances. Find out the specific heat values of common substances and how water's. Heat capacity is the amount of heat needed to raise the temperature of an object by one unit. The si. What Is The Highest Heat Capacity.
From poe.com
What substance possesses the highest heat capacity? Poe What Is The Highest Heat Capacity Specific heat is defined as the amount of heat one gram of a substance must absorb or. Learn what specific heat and heat capacity are, how they are calculated, and what units they use. 55 rows find the volumetric, mass and molar heat capacities of various substances and materials, including air, at different. Heat capacity for a given matter depends. What Is The Highest Heat Capacity.
From www.slideserve.com
PPT 18 Heat and the First Law of Thermodynamics PowerPoint What Is The Highest Heat Capacity Specific heat is defined as the amount of heat one gram of a substance must absorb or. Learn what specific heat and heat capacity are, how they are calculated, and what units they use. Heat capacity is defined as the amount of heat energy required to raise the temperature of a given quantity of matter by one degree celsius. Find. What Is The Highest Heat Capacity.
From www.slideserve.com
PPT Chapter 17 The first law of thermodynamics PowerPoint What Is The Highest Heat Capacity Find out the specific heat values of common substances and how water's. Specific heat is defined as the amount of heat one gram of a substance must absorb or. Heat capacity is defined as the amount of heat energy required to raise the temperature of a given quantity of matter by one degree celsius. The si unit of heat capacity. What Is The Highest Heat Capacity.
From www.slideserve.com
PPT Atmospheric Moisture PowerPoint Presentation, free download ID What Is The Highest Heat Capacity Heat capacity is defined as the amount of heat energy required to raise the temperature of a given quantity of matter by one degree celsius. Specific heat is defined as the amount of heat one gram of a substance must absorb or. Learn what specific heat and heat capacity are, how they are calculated, and what units they use. Heat. What Is The Highest Heat Capacity.
From pressbooks.bccampus.ca
Water has a high heat capacity Chemistry for Biology 1190 Students What Is The Highest Heat Capacity Find out the specific heat values of common substances and how water's. Heat capacity is the amount of heat needed to raise the temperature of an object by one unit. Water has the highest specific heat capacity of any liquid. Specific heat is defined as the amount of heat one gram of a substance must absorb or. The si unit. What Is The Highest Heat Capacity.
From studymind.co.uk
Heat Capacity Study Mind What Is The Highest Heat Capacity The si unit of heat capacity is joule per kelvin (j/k),. Water has the highest specific heat capacity of any liquid. Learn what specific heat and heat capacity are, how they are calculated, and what units they use. Heat capacity is defined as the amount of heat energy required to raise the temperature of a given quantity of matter by. What Is The Highest Heat Capacity.
From www.slideserve.com
PPT Water PowerPoint Presentation, free download ID6095414 What Is The Highest Heat Capacity Specific heat is defined as the amount of heat one gram of a substance must absorb or. Heat capacity for a given matter depends on its size or. Heat capacity is the amount of heat needed to raise the temperature of an object by one unit. 55 rows find the volumetric, mass and molar heat capacities of various substances and. What Is The Highest Heat Capacity.
From klargdopl.blob.core.windows.net
Short Definition Of Heat at Robert Petrin blog What Is The Highest Heat Capacity Find out the specific heat values of common substances and how water's. Heat capacity is defined as the amount of heat energy required to raise the temperature of a given quantity of matter by one degree celsius. Heat capacity is the amount of heat needed to raise the temperature of an object by one unit. The si unit of heat. What Is The Highest Heat Capacity.
From www.tec-science.com
Specific heat capacity of selected substances tecscience What Is The Highest Heat Capacity Heat capacity for a given matter depends on its size or. Specific heat is defined as the amount of heat one gram of a substance must absorb or. The si unit of heat capacity is joule per kelvin (j/k),. Heat capacity is the amount of heat needed to raise the temperature of an object by one unit. Find out the. What Is The Highest Heat Capacity.
From www.youtube.com
Specific Heat and Heat Capacity YouTube What Is The Highest Heat Capacity Find the specific heat of various products, such as metals, liquids, solids, gases, and organic and inorganic substances. The si unit of heat capacity is joule per kelvin (j/k),. Heat capacity for a given matter depends on its size or. Learn what specific heat and heat capacity are, how they are calculated, and what units they use. Heat capacity is. What Is The Highest Heat Capacity.
From www.slideserve.com
PPT Heat Capacity PowerPoint Presentation, free download ID6674197 What Is The Highest Heat Capacity Learn what specific heat and heat capacity are, how they are calculated, and what units they use. Find out the specific heat values of common substances and how water's. Specific heat is defined as the amount of heat one gram of a substance must absorb or. Heat capacity for a given matter depends on its size or. 55 rows find. What Is The Highest Heat Capacity.
From www.nagwa.com
Question Video Understanding Specific Heat Capacity Nagwa What Is The Highest Heat Capacity Water has the highest specific heat capacity of any liquid. Learn what specific heat and heat capacity are, how they are calculated, and what units they use. Heat capacity for a given matter depends on its size or. Find the specific heat of various products, such as metals, liquids, solids, gases, and organic and inorganic substances. The si unit of. What Is The Highest Heat Capacity.
From abramsrewing.blogspot.com
Specific Heat Capacity Definition AbramsrEwing What Is The Highest Heat Capacity Find the specific heat of various products, such as metals, liquids, solids, gases, and organic and inorganic substances. Heat capacity for a given matter depends on its size or. Find out the specific heat values of common substances and how water's. Heat capacity is the amount of heat needed to raise the temperature of an object by one unit. Heat. What Is The Highest Heat Capacity.