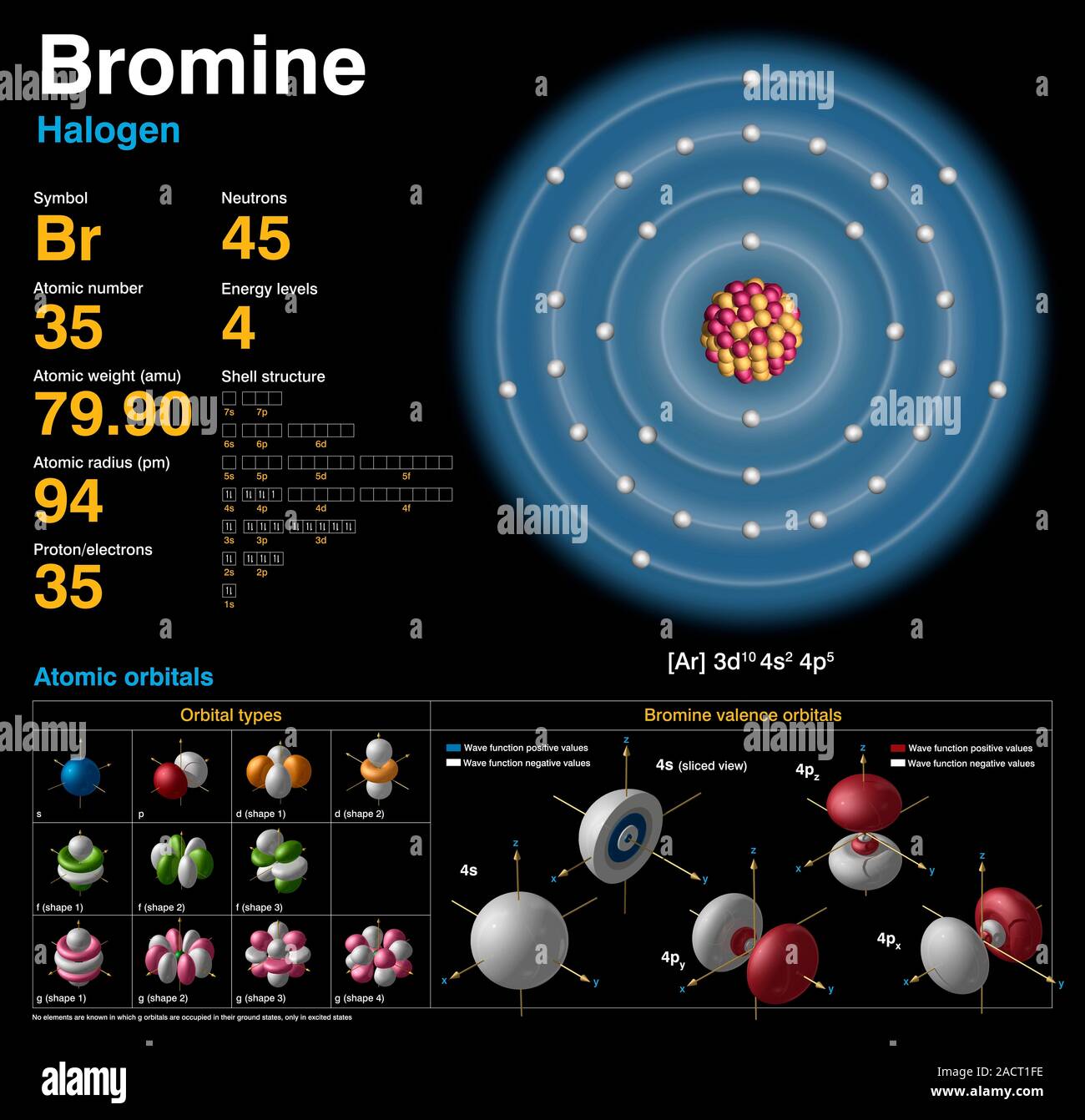
Bromine For Electrons . The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. It was the first element to be extracted. Explore our new interactive periodic table (with rotating bohr models and more) details about this periodic table: In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Also, we will provide the pictures of the same. Bromine has an atomic number of 35, which means that its. Bromine is the 35th element in the periodic table and the symbol is ‘br’. Its properties are thus intermediate between those of chlorine and iodine. Please read the full article below for more information. This can be shortened to #[ar] 4s^2 3d^10 4p^5#.
from www.alamy.com
Bromine has an atomic number of 35, which means that its. Also, we will provide the pictures of the same. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. Bromine is the 35th element in the periodic table and the symbol is ‘br’. Its properties are thus intermediate between those of chlorine and iodine. Please read the full article below for more information. It was the first element to be extracted. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom.
Bromine (Br). Diagram of the nuclear composition, electron configuration, chemical data, and
Bromine For Electrons Also, we will provide the pictures of the same. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. Its properties are thus intermediate between those of chlorine and iodine. Bromine has an atomic number of 35, which means that its. Please read the full article below for more information. Bromine is the 35th element in the periodic table and the symbol is ‘br’. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Also, we will provide the pictures of the same. It was the first element to be extracted. Explore our new interactive periodic table (with rotating bohr models and more) details about this periodic table:
From autoctrls.com
Understanding the Bromine Electron Dot Diagram A Comprehensive Guide Bromine For Electrons Also, we will provide the pictures of the same. Its properties are thus intermediate between those of chlorine and iodine. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. The electron configuration of bromine is #1s^2 2s^2 2p^6. Bromine For Electrons.
From mavink.com
Lewis Dot Diagram For Bromine Bromine For Electrons Bromine has an atomic number of 35, which means that its. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Explore our new interactive periodic table (with rotating bohr models and more) details about this periodic table: Bromine is the 35th element in the periodic table and. Bromine For Electrons.
From www.nuclear-power.com
Bromine Electron Affinity Electronegativity Ionization Energy of Bromine Bromine For Electrons Its properties are thus intermediate between those of chlorine and iodine. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. Explore our new interactive periodic table (with rotating. Bromine For Electrons.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Bromine For Electrons Also, we will provide the pictures of the same. Bromine is the 35th element in the periodic table and the symbol is ‘br’. Explore our new interactive periodic table (with rotating bohr models and more) details about this periodic table: Please read the full article below for more information. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2. Bromine For Electrons.
From www.shutterstock.com
Bohr Model Bromine Atom Electron Structure Stock Vector (Royalty Free) 1957183333 Shutterstock Bromine For Electrons Bromine has an atomic number of 35, which means that its. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10. Bromine For Electrons.
From www.youtube.com
Br electronic configurationHow to write electronic configuration of Bromine YouTube Bromine For Electrons This can be shortened to #[ar] 4s^2 3d^10 4p^5#. It was the first element to be extracted. Its properties are thus intermediate between those of chlorine and iodine. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Bromine is the 35th element in the periodic table and. Bromine For Electrons.
From www.vectorstock.com
Symbol and electron diagram for bromine Royalty Free Vector Bromine For Electrons Explore our new interactive periodic table (with rotating bohr models and more) details about this periodic table: The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. Bromine is. Bromine For Electrons.
From manuallistcantabank.z21.web.core.windows.net
Lewis Dot Diagram For Bromine Bromine For Electrons Please read the full article below for more information. Bromine has an atomic number of 35, which means that its. Explore our new interactive periodic table (with rotating bohr models and more) details about this periodic table: Bromine is the 35th element in the periodic table and the symbol is ‘br’. Its properties are thus intermediate between those of chlorine. Bromine For Electrons.
From utedzz.blogspot.com
Bromine Periodic Table Square Periodic Table Timeline Bromine For Electrons Explore our new interactive periodic table (with rotating bohr models and more) details about this periodic table: This can be shortened to #[ar] 4s^2 3d^10 4p^5#. It was the first element to be extracted. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Bromine has an atomic. Bromine For Electrons.
From periodictable.me
How Do We Find The Electron Configuration For Bromine Dynamic Periodic Table of Elements and Bromine For Electrons It was the first element to be extracted. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. Also, we will provide the pictures of the same. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. In this article today we are going. Bromine For Electrons.
From www.youtube.com
How to Find the Valence Electrons for Bromine (Br) YouTube Bromine For Electrons Explore our new interactive periodic table (with rotating bohr models and more) details about this periodic table: Also, we will provide the pictures of the same. It was the first element to be extracted. Please read the full article below for more information. In this article today we are going to tell you about the electron configuration of bromine, its. Bromine For Electrons.
From material-properties.org
Bromine Protons Neutrons Electrons Electron Configuration Bromine For Electrons In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Its properties are thus intermediate between those of chlorine and iodine. Bromine has an atomic number of 35, which means that its. Bromine is the 35th element in the periodic table and the symbol is ‘br’. It was. Bromine For Electrons.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron configuration, and valence orbitals Bromine For Electrons Explore our new interactive periodic table (with rotating bohr models and more) details about this periodic table: The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. Bromine is the 35th element in the periodic table and the symbol is ‘br’. Please read. Bromine For Electrons.
From ar.inspiredpencil.com
Atomic Structure Of Bromine Bromine For Electrons In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Bromine is the 35th element in the periodic table and the symbol is ‘br’. Explore our new interactive periodic table (with rotating bohr models and more) details about this periodic table: The electron configuration of bromine is #1s^2. Bromine For Electrons.
From www.alamy.com
Symbol and electron diagram for Bromine illustration Stock Vector Image & Art Alamy Bromine For Electrons Please read the full article below for more information. Explore our new interactive periodic table (with rotating bohr models and more) details about this periodic table: The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and. Bromine For Electrons.
From www.shutterstock.com
Symbol Electron Diagram Bromine Illustration Stock Vector (Royalty Free) 318671348 Bromine For Electrons It was the first element to be extracted. Its properties are thus intermediate between those of chlorine and iodine. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. Bromine is the 35th element in the periodic table and the symbol is ‘br’.. Bromine For Electrons.
From www.alamy.com
3d render of atom structure of bromine isolated over white background Protons are represented as Bromine For Electrons This can be shortened to #[ar] 4s^2 3d^10 4p^5#. Its properties are thus intermediate between those of chlorine and iodine. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. It was the first element to be extracted. Also, we will provide the pictures of the same. Please. Bromine For Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Bromine For Electrons The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. Also, we will provide the pictures of the same. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Bromine is the 35th element in the periodic table and the symbol is ‘br’. This. Bromine For Electrons.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron configuration, chemical data, and Bromine For Electrons The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. Bromine has an atomic number of 35, which means that its. Bromine is the 35th element in the periodic table and the symbol is ‘br’. Please read the full article below for more. Bromine For Electrons.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine For Electrons Also, we will provide the pictures of the same. Explore our new interactive periodic table (with rotating bohr models and more) details about this periodic table: It was the first element to be extracted. Bromine has an atomic number of 35, which means that its. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6. Bromine For Electrons.
From sciencenotes.org
Bromine Facts Atomic Number 35 and Element Symbol Br Bromine For Electrons Please read the full article below for more information. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of electrons within the atom. Also,. Bromine For Electrons.
From www.youtube.com
How to find the Number of Protons, Electrons, Neutrons for Bromine (Br) YouTube Bromine For Electrons The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. Also, we will provide the pictures of the same. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. Please read the full article below for more information. It was the first element to be extracted. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2. Bromine For Electrons.
From ar.inspiredpencil.com
Electron Configuration For Bromine Bromine For Electrons Its properties are thus intermediate between those of chlorine and iodine. It was the first element to be extracted. Also, we will provide the pictures of the same. Explore our new interactive periodic table (with rotating bohr models and more) details about this periodic table: This can be shortened to #[ar] 4s^2 3d^10 4p^5#. Please read the full article below. Bromine For Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Bromine For Electrons Also, we will provide the pictures of the same. Bromine is the 35th element in the periodic table and the symbol is ‘br’. Bromine has an atomic number of 35, which means that its. Its properties are thus intermediate between those of chlorine and iodine. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. The bromine electron configuration, denoted as. Bromine For Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Bromine (Br)? Bromine For Electrons It was the first element to be extracted. Bromine has an atomic number of 35, which means that its. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. Bromine is the 35th element in the periodic table and the symbol is ‘br’. Also, we will provide the pictures of the same. The bromine electron configuration,. Bromine For Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Bromine For Electrons The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. Its properties are thus intermediate between those of chlorine and iodine. It was the first element to be extracted. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. Also, we will provide the pictures of the same. Bromine has an atomic number of 35, which means. Bromine For Electrons.
From www.dreamstime.com
Atom of Bromine with Detailed Core and Its 35 Electrons on Black Stock Illustration Bromine For Electrons Please read the full article below for more information. It was the first element to be extracted. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. Its properties are thus intermediate between those of chlorine and iodine. Explore. Bromine For Electrons.
From material-properties.org
Bromine Protons Neutrons Electrons Electron Configuration Bromine For Electrons Bromine is the 35th element in the periodic table and the symbol is ‘br’. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. Bromine has an atomic number of 35, which means that its. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise. Bromine For Electrons.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Bromine For Electrons Bromine is the 35th element in the periodic table and the symbol is ‘br’. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Also, we will provide the pictures of the same. It was the first element to be extracted. The electron configuration of bromine is #1s^2. Bromine For Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Bromine For Electrons It was the first element to be extracted. Explore our new interactive periodic table (with rotating bohr models and more) details about this periodic table: Please read the full article below for more information. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2. Bromine For Electrons.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine For Electrons Please read the full article below for more information. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. Also, we will provide the pictures of the same. Explore our new interactive periodic table (with rotating bohr models and more) details about this periodic table: This can be shortened to #[ar] 4s^2 3d^10 4p^5#. Bromine is. Bromine For Electrons.
From ar.inspiredpencil.com
Bromine Valence Electrons Bromine For Electrons Explore our new interactive periodic table (with rotating bohr models and more) details about this periodic table: The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. Bromine is the 35th element in the periodic table and the symbol is ‘br’. Its properties are thus intermediate between those of chlorine and iodine. It was the first. Bromine For Electrons.
From stock.adobe.com
Br Bromine Element Information Facts, Properties, Trends, Uses and comparison Periodic Table Bromine For Electrons The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. Bromine is the 35th element in the periodic table and the symbol is ‘br’. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. The bromine electron configuration, denoted as 4s2 3d10 4p5 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5, showcases the precise placement of. Bromine For Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Bromine For Electrons Explore our new interactive periodic table (with rotating bohr models and more) details about this periodic table: Bromine has an atomic number of 35, which means that its. Also, we will provide the pictures of the same. The electron configuration of bromine is #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^5#. This can be shortened to #[ar] 4s^2 3d^10 4p^5#.. Bromine For Electrons.
From www.dreamstime.com
Bromine Atom, with Mass and Energy Levels. Stock Vector Illustration of nucleus, particle Bromine For Electrons Explore our new interactive periodic table (with rotating bohr models and more) details about this periodic table: Also, we will provide the pictures of the same. Please read the full article below for more information. It was the first element to be extracted. This can be shortened to #[ar] 4s^2 3d^10 4p^5#. Bromine is the 35th element in the periodic. Bromine For Electrons.