Why Is Platinum Used In Electrochemical Cells . The cell consists of hydrochloric acid, hydrogen gas and uses platinum electrodes. Researchers elucidate mechanisms for controlling the surface oxidation processes that affect the performance of platinum catalysts in alkaline media. The electrode can be made from an inert, highly conducting metal such as platinum to prevent it from reacting during a redox process, where it does not appear in the. These are very useful as they are metallic, so will conduct. As the hydrogen gas flows over the porous platinum, an equilibrium is set up between hydrogen molecules and hydrogen ions in. The platinum surface has to be specially treated by. This makes it easier to. The latter is seldom used in routine electrochemical measurements because it is more difficult to prepare;
from schoolbag.info
As the hydrogen gas flows over the porous platinum, an equilibrium is set up between hydrogen molecules and hydrogen ions in. This makes it easier to. Researchers elucidate mechanisms for controlling the surface oxidation processes that affect the performance of platinum catalysts in alkaline media. The electrode can be made from an inert, highly conducting metal such as platinum to prevent it from reacting during a redox process, where it does not appear in the. The latter is seldom used in routine electrochemical measurements because it is more difficult to prepare; The cell consists of hydrochloric acid, hydrogen gas and uses platinum electrodes. The platinum surface has to be specially treated by. These are very useful as they are metallic, so will conduct.
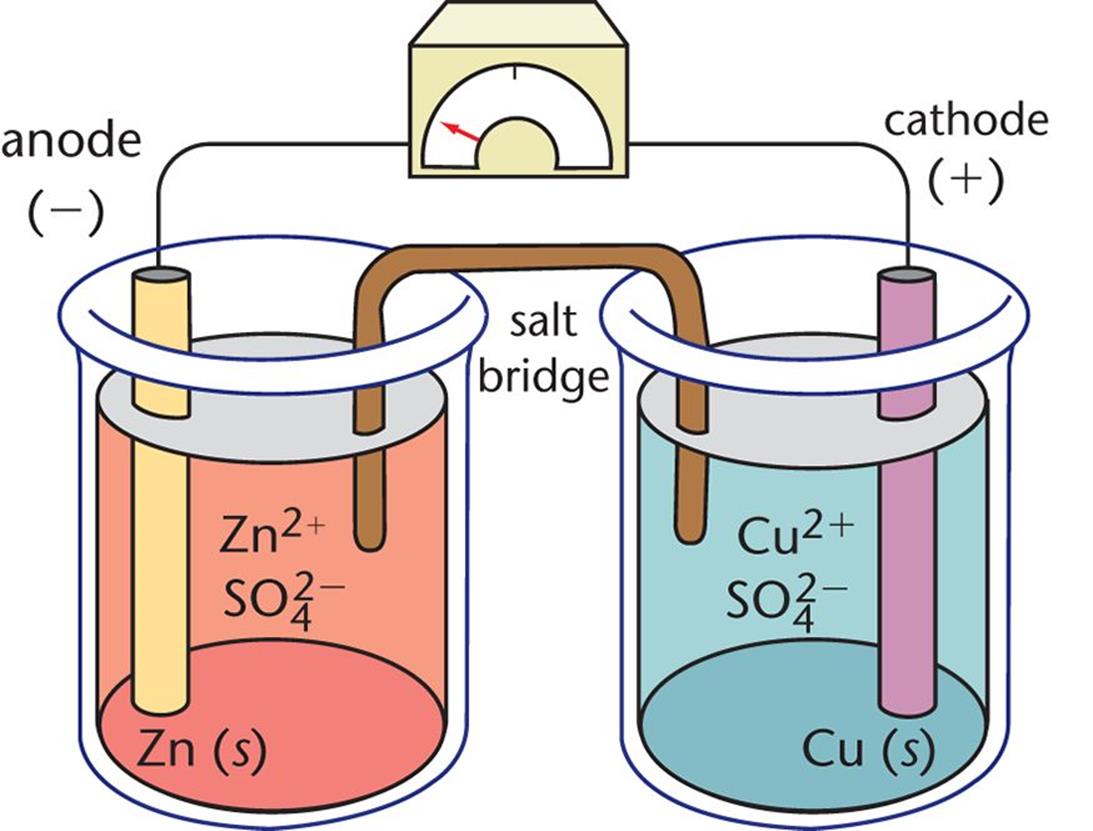
Figure 12.1. Daniell Cell In this galvanic cell, zinc is the anode and copper is the cathode
Why Is Platinum Used In Electrochemical Cells The electrode can be made from an inert, highly conducting metal such as platinum to prevent it from reacting during a redox process, where it does not appear in the. These are very useful as they are metallic, so will conduct. This makes it easier to. The latter is seldom used in routine electrochemical measurements because it is more difficult to prepare; As the hydrogen gas flows over the porous platinum, an equilibrium is set up between hydrogen molecules and hydrogen ions in. The cell consists of hydrochloric acid, hydrogen gas and uses platinum electrodes. The electrode can be made from an inert, highly conducting metal such as platinum to prevent it from reacting during a redox process, where it does not appear in the. The platinum surface has to be specially treated by. Researchers elucidate mechanisms for controlling the surface oxidation processes that affect the performance of platinum catalysts in alkaline media.
From www.researchgate.net
(PDF) Electrochemical Cells An Introduction Why Is Platinum Used In Electrochemical Cells This makes it easier to. The cell consists of hydrochloric acid, hydrogen gas and uses platinum electrodes. The platinum surface has to be specially treated by. Researchers elucidate mechanisms for controlling the surface oxidation processes that affect the performance of platinum catalysts in alkaline media. The latter is seldom used in routine electrochemical measurements because it is more difficult to. Why Is Platinum Used In Electrochemical Cells.
From leah4sci.com
Electrochemistry Galvanic / Voltaic and Electrolytic Cells MCAT and Organic Chemistry Study Why Is Platinum Used In Electrochemical Cells These are very useful as they are metallic, so will conduct. The cell consists of hydrochloric acid, hydrogen gas and uses platinum electrodes. Researchers elucidate mechanisms for controlling the surface oxidation processes that affect the performance of platinum catalysts in alkaline media. The latter is seldom used in routine electrochemical measurements because it is more difficult to prepare; The platinum. Why Is Platinum Used In Electrochemical Cells.
From www.thoughtco.com
Electrochemical Cell Definition Why Is Platinum Used In Electrochemical Cells The platinum surface has to be specially treated by. As the hydrogen gas flows over the porous platinum, an equilibrium is set up between hydrogen molecules and hydrogen ions in. The cell consists of hydrochloric acid, hydrogen gas and uses platinum electrodes. Researchers elucidate mechanisms for controlling the surface oxidation processes that affect the performance of platinum catalysts in alkaline. Why Is Platinum Used In Electrochemical Cells.
From www.researchgate.net
What reaction occurs at the platinum counter electrode in nonaqueous 3electrode experiments? Why Is Platinum Used In Electrochemical Cells Researchers elucidate mechanisms for controlling the surface oxidation processes that affect the performance of platinum catalysts in alkaline media. The latter is seldom used in routine electrochemical measurements because it is more difficult to prepare; This makes it easier to. These are very useful as they are metallic, so will conduct. The cell consists of hydrochloric acid, hydrogen gas and. Why Is Platinum Used In Electrochemical Cells.
From saylordotorg.github.io
Electrochemistry Why Is Platinum Used In Electrochemical Cells This makes it easier to. The platinum surface has to be specially treated by. As the hydrogen gas flows over the porous platinum, an equilibrium is set up between hydrogen molecules and hydrogen ions in. The electrode can be made from an inert, highly conducting metal such as platinum to prevent it from reacting during a redox process, where it. Why Is Platinum Used In Electrochemical Cells.
From www.researchgate.net
Schematic representation of the electrochemical cell used in this... Download Scientific Diagram Why Is Platinum Used In Electrochemical Cells The cell consists of hydrochloric acid, hydrogen gas and uses platinum electrodes. These are very useful as they are metallic, so will conduct. As the hydrogen gas flows over the porous platinum, an equilibrium is set up between hydrogen molecules and hydrogen ions in. The platinum surface has to be specially treated by. The electrode can be made from an. Why Is Platinum Used In Electrochemical Cells.
From manuallistcantabank.z21.web.core.windows.net
Electrochemical Cell Diagram Why Is Platinum Used In Electrochemical Cells Researchers elucidate mechanisms for controlling the surface oxidation processes that affect the performance of platinum catalysts in alkaline media. As the hydrogen gas flows over the porous platinum, an equilibrium is set up between hydrogen molecules and hydrogen ions in. These are very useful as they are metallic, so will conduct. The platinum surface has to be specially treated by.. Why Is Platinum Used In Electrochemical Cells.
From chemistryuntold.com
TYPES OF ELECTROCHEMICAL CELLS Why Is Platinum Used In Electrochemical Cells Researchers elucidate mechanisms for controlling the surface oxidation processes that affect the performance of platinum catalysts in alkaline media. The platinum surface has to be specially treated by. These are very useful as they are metallic, so will conduct. The electrode can be made from an inert, highly conducting metal such as platinum to prevent it from reacting during a. Why Is Platinum Used In Electrochemical Cells.
From www.sigmaaldrich.cn
Electrochemistry on the Bench and in the Field Why Is Platinum Used In Electrochemical Cells The cell consists of hydrochloric acid, hydrogen gas and uses platinum electrodes. These are very useful as they are metallic, so will conduct. This makes it easier to. The latter is seldom used in routine electrochemical measurements because it is more difficult to prepare; Researchers elucidate mechanisms for controlling the surface oxidation processes that affect the performance of platinum catalysts. Why Is Platinum Used In Electrochemical Cells.
From www.vrogue.co
Standard Notation For Electrochemical Cells vrogue.co Why Is Platinum Used In Electrochemical Cells The latter is seldom used in routine electrochemical measurements because it is more difficult to prepare; The platinum surface has to be specially treated by. As the hydrogen gas flows over the porous platinum, an equilibrium is set up between hydrogen molecules and hydrogen ions in. These are very useful as they are metallic, so will conduct. The cell consists. Why Is Platinum Used In Electrochemical Cells.
From courses.lumenlearning.com
17.3 Standard Reduction Potentials General College Chemistry II Why Is Platinum Used In Electrochemical Cells The electrode can be made from an inert, highly conducting metal such as platinum to prevent it from reacting during a redox process, where it does not appear in the. The latter is seldom used in routine electrochemical measurements because it is more difficult to prepare; This makes it easier to. The platinum surface has to be specially treated by.. Why Is Platinum Used In Electrochemical Cells.
From courses.lumenlearning.com
Galvanic Cells Chemistry Atoms First Why Is Platinum Used In Electrochemical Cells These are very useful as they are metallic, so will conduct. This makes it easier to. Researchers elucidate mechanisms for controlling the surface oxidation processes that affect the performance of platinum catalysts in alkaline media. The latter is seldom used in routine electrochemical measurements because it is more difficult to prepare; As the hydrogen gas flows over the porous platinum,. Why Is Platinum Used In Electrochemical Cells.
From chem.libretexts.org
11.1 Galvanic Cells Chemistry LibreTexts Why Is Platinum Used In Electrochemical Cells As the hydrogen gas flows over the porous platinum, an equilibrium is set up between hydrogen molecules and hydrogen ions in. Researchers elucidate mechanisms for controlling the surface oxidation processes that affect the performance of platinum catalysts in alkaline media. The electrode can be made from an inert, highly conducting metal such as platinum to prevent it from reacting during. Why Is Platinum Used In Electrochemical Cells.
From pediaa.com
What is the Difference Between Oxidation and Reduction Electrochemical Reactions Why Is Platinum Used In Electrochemical Cells The platinum surface has to be specially treated by. The cell consists of hydrochloric acid, hydrogen gas and uses platinum electrodes. As the hydrogen gas flows over the porous platinum, an equilibrium is set up between hydrogen molecules and hydrogen ions in. This makes it easier to. The electrode can be made from an inert, highly conducting metal such as. Why Is Platinum Used In Electrochemical Cells.
From rohanfersmorrison.blogspot.com
Identify the Conditions for a Standard Electrochemical Cell. Why Is Platinum Used In Electrochemical Cells These are very useful as they are metallic, so will conduct. This makes it easier to. The electrode can be made from an inert, highly conducting metal such as platinum to prevent it from reacting during a redox process, where it does not appear in the. The cell consists of hydrochloric acid, hydrogen gas and uses platinum electrodes. The latter. Why Is Platinum Used In Electrochemical Cells.
From www.youtube.com
Half cell, oxidation half cell,reduction half cell(Electrochemistry part 6 CBSE class 12 YouTube Why Is Platinum Used In Electrochemical Cells As the hydrogen gas flows over the porous platinum, an equilibrium is set up between hydrogen molecules and hydrogen ions in. These are very useful as they are metallic, so will conduct. Researchers elucidate mechanisms for controlling the surface oxidation processes that affect the performance of platinum catalysts in alkaline media. The platinum surface has to be specially treated by.. Why Is Platinum Used In Electrochemical Cells.
From schoolbag.info
Figure 12.1. Daniell Cell In this galvanic cell, zinc is the anode and copper is the cathode Why Is Platinum Used In Electrochemical Cells The latter is seldom used in routine electrochemical measurements because it is more difficult to prepare; This makes it easier to. The electrode can be made from an inert, highly conducting metal such as platinum to prevent it from reacting during a redox process, where it does not appear in the. The cell consists of hydrochloric acid, hydrogen gas and. Why Is Platinum Used In Electrochemical Cells.
From wisc.pb.unizin.org
D40.3 Standard HalfCell Potentials Chemistry 109 Fall 2021 Why Is Platinum Used In Electrochemical Cells As the hydrogen gas flows over the porous platinum, an equilibrium is set up between hydrogen molecules and hydrogen ions in. The cell consists of hydrochloric acid, hydrogen gas and uses platinum electrodes. Researchers elucidate mechanisms for controlling the surface oxidation processes that affect the performance of platinum catalysts in alkaline media. These are very useful as they are metallic,. Why Is Platinum Used In Electrochemical Cells.
From learningkurugon1.z22.web.core.windows.net
Electrochemical Cells Gcse Chemistry Why Is Platinum Used In Electrochemical Cells The cell consists of hydrochloric acid, hydrogen gas and uses platinum electrodes. As the hydrogen gas flows over the porous platinum, an equilibrium is set up between hydrogen molecules and hydrogen ions in. These are very useful as they are metallic, so will conduct. The latter is seldom used in routine electrochemical measurements because it is more difficult to prepare;. Why Is Platinum Used In Electrochemical Cells.
From www.expii.com
Electrochemical Cell — Definition & Overview Expii Why Is Platinum Used In Electrochemical Cells The platinum surface has to be specially treated by. Researchers elucidate mechanisms for controlling the surface oxidation processes that affect the performance of platinum catalysts in alkaline media. The cell consists of hydrochloric acid, hydrogen gas and uses platinum electrodes. The electrode can be made from an inert, highly conducting metal such as platinum to prevent it from reacting during. Why Is Platinum Used In Electrochemical Cells.
From stoplearn.com
Electrochemical Cells 2023 Why Is Platinum Used In Electrochemical Cells As the hydrogen gas flows over the porous platinum, an equilibrium is set up between hydrogen molecules and hydrogen ions in. Researchers elucidate mechanisms for controlling the surface oxidation processes that affect the performance of platinum catalysts in alkaline media. The platinum surface has to be specially treated by. The cell consists of hydrochloric acid, hydrogen gas and uses platinum. Why Is Platinum Used In Electrochemical Cells.
From courses.lumenlearning.com
Electrolysis Chemistry Why Is Platinum Used In Electrochemical Cells The electrode can be made from an inert, highly conducting metal such as platinum to prevent it from reacting during a redox process, where it does not appear in the. As the hydrogen gas flows over the porous platinum, an equilibrium is set up between hydrogen molecules and hydrogen ions in. Researchers elucidate mechanisms for controlling the surface oxidation processes. Why Is Platinum Used In Electrochemical Cells.
From courses.lumenlearning.com
17.3 Standard Reduction Potentials Chemistry Why Is Platinum Used In Electrochemical Cells The platinum surface has to be specially treated by. The latter is seldom used in routine electrochemical measurements because it is more difficult to prepare; The cell consists of hydrochloric acid, hydrogen gas and uses platinum electrodes. This makes it easier to. As the hydrogen gas flows over the porous platinum, an equilibrium is set up between hydrogen molecules and. Why Is Platinum Used In Electrochemical Cells.
From www.yaclass.in
Types of Electrochemical Cell and Electrolytic Cell — lesson. Science State Board, Class 9. Why Is Platinum Used In Electrochemical Cells The platinum surface has to be specially treated by. These are very useful as they are metallic, so will conduct. This makes it easier to. The latter is seldom used in routine electrochemical measurements because it is more difficult to prepare; The cell consists of hydrochloric acid, hydrogen gas and uses platinum electrodes. As the hydrogen gas flows over the. Why Is Platinum Used In Electrochemical Cells.
From mavink.com
Electrochemical Cell Diagram Why Is Platinum Used In Electrochemical Cells These are very useful as they are metallic, so will conduct. The electrode can be made from an inert, highly conducting metal such as platinum to prevent it from reacting during a redox process, where it does not appear in the. The platinum surface has to be specially treated by. As the hydrogen gas flows over the porous platinum, an. Why Is Platinum Used In Electrochemical Cells.
From 2012books.lardbucket.org
Electrochemistry Why Is Platinum Used In Electrochemical Cells This makes it easier to. These are very useful as they are metallic, so will conduct. As the hydrogen gas flows over the porous platinum, an equilibrium is set up between hydrogen molecules and hydrogen ions in. The electrode can be made from an inert, highly conducting metal such as platinum to prevent it from reacting during a redox process,. Why Is Platinum Used In Electrochemical Cells.
From mmerevise.co.uk
Electrochemical Cells MME Why Is Platinum Used In Electrochemical Cells The electrode can be made from an inert, highly conducting metal such as platinum to prevent it from reacting during a redox process, where it does not appear in the. This makes it easier to. As the hydrogen gas flows over the porous platinum, an equilibrium is set up between hydrogen molecules and hydrogen ions in. These are very useful. Why Is Platinum Used In Electrochemical Cells.
From www.slideserve.com
PPT Electrochemical cells PowerPoint Presentation, free download ID1837944 Why Is Platinum Used In Electrochemical Cells This makes it easier to. The platinum surface has to be specially treated by. Researchers elucidate mechanisms for controlling the surface oxidation processes that affect the performance of platinum catalysts in alkaline media. These are very useful as they are metallic, so will conduct. The cell consists of hydrochloric acid, hydrogen gas and uses platinum electrodes. As the hydrogen gas. Why Is Platinum Used In Electrochemical Cells.
From in.pinterest.com
What is Electrochemical Cell Notation Line notation Cell Diagram Cell diagram Why Is Platinum Used In Electrochemical Cells The platinum surface has to be specially treated by. The latter is seldom used in routine electrochemical measurements because it is more difficult to prepare; As the hydrogen gas flows over the porous platinum, an equilibrium is set up between hydrogen molecules and hydrogen ions in. Researchers elucidate mechanisms for controlling the surface oxidation processes that affect the performance of. Why Is Platinum Used In Electrochemical Cells.
From www.chegg.com
Solved An electrochemical cell is composed of a platinum Why Is Platinum Used In Electrochemical Cells The platinum surface has to be specially treated by. The latter is seldom used in routine electrochemical measurements because it is more difficult to prepare; This makes it easier to. The cell consists of hydrochloric acid, hydrogen gas and uses platinum electrodes. Researchers elucidate mechanisms for controlling the surface oxidation processes that affect the performance of platinum catalysts in alkaline. Why Is Platinum Used In Electrochemical Cells.
From www.slideserve.com
PPT ELECTROCHEMICAL CELLS PowerPoint Presentation, free download ID2068416 Why Is Platinum Used In Electrochemical Cells The latter is seldom used in routine electrochemical measurements because it is more difficult to prepare; The cell consists of hydrochloric acid, hydrogen gas and uses platinum electrodes. The platinum surface has to be specially treated by. Researchers elucidate mechanisms for controlling the surface oxidation processes that affect the performance of platinum catalysts in alkaline media. The electrode can be. Why Is Platinum Used In Electrochemical Cells.
From www.showme.com
Electrochemical cell notation Science, Chemistry ShowMe Why Is Platinum Used In Electrochemical Cells As the hydrogen gas flows over the porous platinum, an equilibrium is set up between hydrogen molecules and hydrogen ions in. Researchers elucidate mechanisms for controlling the surface oxidation processes that affect the performance of platinum catalysts in alkaline media. The cell consists of hydrochloric acid, hydrogen gas and uses platinum electrodes. This makes it easier to. These are very. Why Is Platinum Used In Electrochemical Cells.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Why Is Platinum Used In Electrochemical Cells The latter is seldom used in routine electrochemical measurements because it is more difficult to prepare; Researchers elucidate mechanisms for controlling the surface oxidation processes that affect the performance of platinum catalysts in alkaline media. The cell consists of hydrochloric acid, hydrogen gas and uses platinum electrodes. These are very useful as they are metallic, so will conduct. This makes. Why Is Platinum Used In Electrochemical Cells.
From www.researchgate.net
Figure The anode and cathode reactions in typical electrolytic... Download Scientific Diagram Why Is Platinum Used In Electrochemical Cells This makes it easier to. Researchers elucidate mechanisms for controlling the surface oxidation processes that affect the performance of platinum catalysts in alkaline media. The latter is seldom used in routine electrochemical measurements because it is more difficult to prepare; The cell consists of hydrochloric acid, hydrogen gas and uses platinum electrodes. The electrode can be made from an inert,. Why Is Platinum Used In Electrochemical Cells.
From mavink.com
Electrochemical Cell Diagram Why Is Platinum Used In Electrochemical Cells The latter is seldom used in routine electrochemical measurements because it is more difficult to prepare; The cell consists of hydrochloric acid, hydrogen gas and uses platinum electrodes. As the hydrogen gas flows over the porous platinum, an equilibrium is set up between hydrogen molecules and hydrogen ions in. The platinum surface has to be specially treated by. Researchers elucidate. Why Is Platinum Used In Electrochemical Cells.