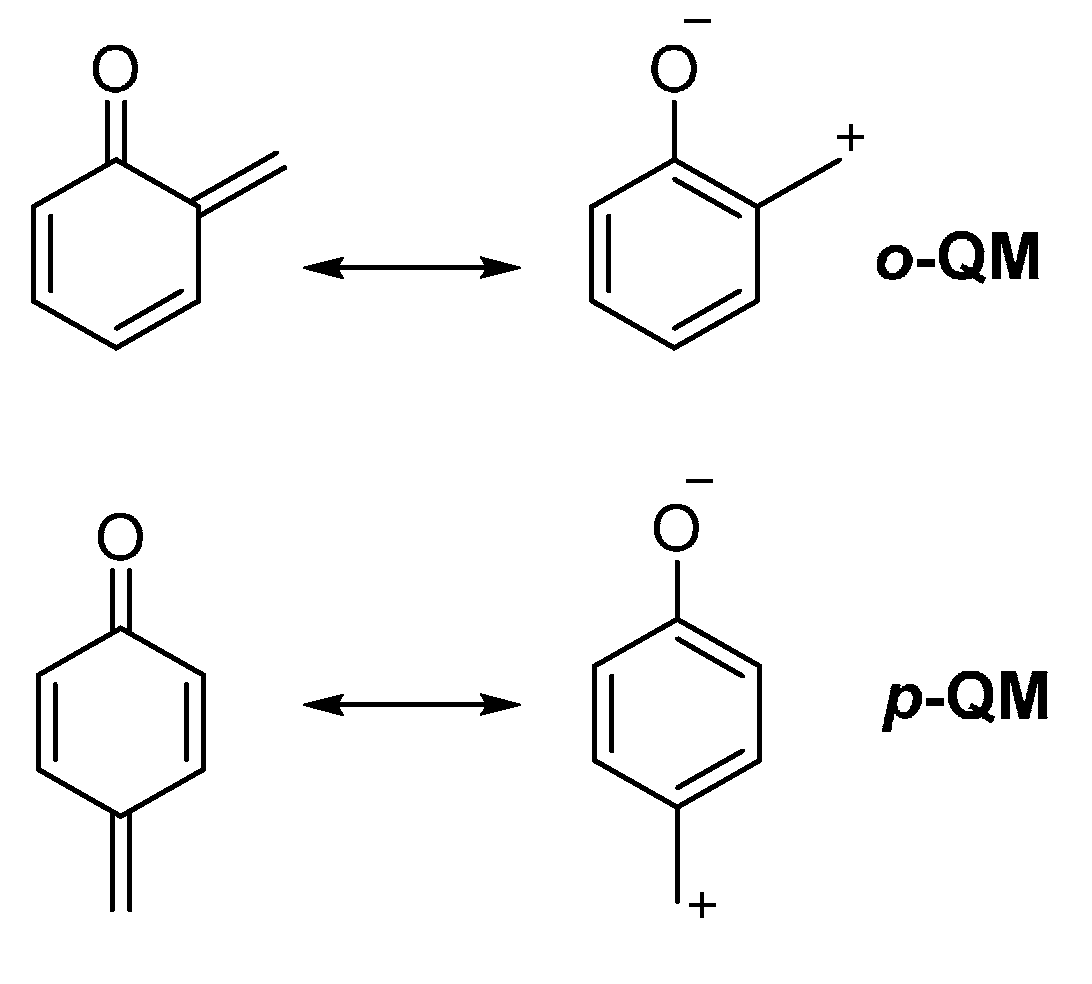
O-Quinolinone Quinone Methide . 1) have been widely used in organic synthesis since the initial report by fries et al. Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. This reaction is highly selective and proceeds smoothly under aqueous conditions.
from www.mdpi.com
Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. This reaction is highly selective and proceeds smoothly under aqueous conditions. 1) have been widely used in organic synthesis since the initial report by fries et al.
Molecules Free FullText The Emergence of Quinone Methides in
O-Quinolinone Quinone Methide This reaction is highly selective and proceeds smoothly under aqueous conditions. Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. 1) have been widely used in organic synthesis since the initial report by fries et al. This reaction is highly selective and proceeds smoothly under aqueous conditions.
From www.semanticscholar.org
Table 1 from Mild and rapid method for the generation of oquinone O-Quinolinone Quinone Methide Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. 1) have been widely used in organic synthesis since the initial report by fries et al. This reaction is highly selective and proceeds smoothly under aqueous conditions. O-Quinolinone Quinone Methide.
From www.mdpi.com
IJMS Free FullText Reactivities of Quinone Methides versus o O-Quinolinone Quinone Methide 1) have been widely used in organic synthesis since the initial report by fries et al. This reaction is highly selective and proceeds smoothly under aqueous conditions. Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. O-Quinolinone Quinone Methide.
From pubs.rsc.org
ortho Quinone methide ( o QM) a highly reactive, ephemeral and O-Quinolinone Quinone Methide Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. 1) have been widely used in organic synthesis since the initial report by fries et al. This reaction is highly selective and proceeds smoothly under aqueous conditions. O-Quinolinone Quinone Methide.
From pubs.acs.org
Harnessing orthoQuinone Methides in Natural Product Biosynthesis and O-Quinolinone Quinone Methide Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. This reaction is highly selective and proceeds smoothly under aqueous conditions. 1) have been widely used in organic synthesis since the initial report by fries et al. O-Quinolinone Quinone Methide.
From www.researchgate.net
Scheme 2 Reaction of oquinone methide with benzamide. Download O-Quinolinone Quinone Methide 1) have been widely used in organic synthesis since the initial report by fries et al. This reaction is highly selective and proceeds smoothly under aqueous conditions. Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. O-Quinolinone Quinone Methide.
From pubs.rsc.org
orthoQuinone methide (oQM) a highly reactive, ephemeral and O-Quinolinone Quinone Methide This reaction is highly selective and proceeds smoothly under aqueous conditions. Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. 1) have been widely used in organic synthesis since the initial report by fries et al. O-Quinolinone Quinone Methide.
From www.researchgate.net
Quinone methide formation of 8−10 , 8−5 , and 8−12 homocoupled O-Quinolinone Quinone Methide 1) have been widely used in organic synthesis since the initial report by fries et al. Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. This reaction is highly selective and proceeds smoothly under aqueous conditions. O-Quinolinone Quinone Methide.
From www.semanticscholar.org
Chemoenzymatic oQuinone Methide Formation. Semantic Scholar O-Quinolinone Quinone Methide 1) have been widely used in organic synthesis since the initial report by fries et al. This reaction is highly selective and proceeds smoothly under aqueous conditions. Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. O-Quinolinone Quinone Methide.
From www.mdpi.com
IJMS Free FullText Reactivities of Quinone Methides versus o O-Quinolinone Quinone Methide This reaction is highly selective and proceeds smoothly under aqueous conditions. 1) have been widely used in organic synthesis since the initial report by fries et al. Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. O-Quinolinone Quinone Methide.
From www.researchgate.net
Quinone methide formation and rearomatization reactions of coniferyl O-Quinolinone Quinone Methide This reaction is highly selective and proceeds smoothly under aqueous conditions. Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. 1) have been widely used in organic synthesis since the initial report by fries et al. O-Quinolinone Quinone Methide.
From www.researchgate.net
Quinone methide formation and rearomatization reactions of coniferyl O-Quinolinone Quinone Methide Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. This reaction is highly selective and proceeds smoothly under aqueous conditions. 1) have been widely used in organic synthesis since the initial report by fries et al. O-Quinolinone Quinone Methide.
From www.mdpi.com
IJMS Free FullText Reactivities of Quinone Methides versus o O-Quinolinone Quinone Methide This reaction is highly selective and proceeds smoothly under aqueous conditions. Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. 1) have been widely used in organic synthesis since the initial report by fries et al. O-Quinolinone Quinone Methide.
From pubs.rsc.org
orthoQuinone methide (oQM) a highly reactive, ephemeral and O-Quinolinone Quinone Methide 1) have been widely used in organic synthesis since the initial report by fries et al. This reaction is highly selective and proceeds smoothly under aqueous conditions. Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. O-Quinolinone Quinone Methide.
From www.semanticscholar.org
Table 1 from An Oxyanion Accelerated [1,5]oQuinone Methide Shift O-Quinolinone Quinone Methide This reaction is highly selective and proceeds smoothly under aqueous conditions. 1) have been widely used in organic synthesis since the initial report by fries et al. Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. O-Quinolinone Quinone Methide.
From www.researchgate.net
Scheme 2 Reaction of oquinone methide with benzamide. Download O-Quinolinone Quinone Methide Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. This reaction is highly selective and proceeds smoothly under aqueous conditions. 1) have been widely used in organic synthesis since the initial report by fries et al. O-Quinolinone Quinone Methide.
From www.mdpi.com
IJMS Free FullText Reactivities of Quinone Methides versus o O-Quinolinone Quinone Methide 1) have been widely used in organic synthesis since the initial report by fries et al. Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. This reaction is highly selective and proceeds smoothly under aqueous conditions. O-Quinolinone Quinone Methide.
From achs-prod.acs.org
oQuinone Methides and oQuinone Sulfides via Arynes Synthesis of O-Quinolinone Quinone Methide Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. 1) have been widely used in organic synthesis since the initial report by fries et al. This reaction is highly selective and proceeds smoothly under aqueous conditions. O-Quinolinone Quinone Methide.
From pubs.acs.org
A Bioorthogonal Ligation Enabled by Click Cycloaddition of o O-Quinolinone Quinone Methide Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. This reaction is highly selective and proceeds smoothly under aqueous conditions. 1) have been widely used in organic synthesis since the initial report by fries et al. O-Quinolinone Quinone Methide.
From www.mdpi.com
IJMS Free FullText Reactivities of Quinone Methides versus o O-Quinolinone Quinone Methide This reaction is highly selective and proceeds smoothly under aqueous conditions. 1) have been widely used in organic synthesis since the initial report by fries et al. Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. O-Quinolinone Quinone Methide.
From lookfordiagnosis.com
Salacia O-Quinolinone Quinone Methide Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. 1) have been widely used in organic synthesis since the initial report by fries et al. This reaction is highly selective and proceeds smoothly under aqueous conditions. O-Quinolinone Quinone Methide.
From www.researchgate.net
Photoelectron spectra for the C 7 H 6 O isomers o quinone methide, p O-Quinolinone Quinone Methide Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. This reaction is highly selective and proceeds smoothly under aqueous conditions. 1) have been widely used in organic synthesis since the initial report by fries et al. O-Quinolinone Quinone Methide.
From www.researchgate.net
Structural features of different quinone methides (QMs). Download O-Quinolinone Quinone Methide This reaction is highly selective and proceeds smoothly under aqueous conditions. 1) have been widely used in organic synthesis since the initial report by fries et al. Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. O-Quinolinone Quinone Methide.
From www.researchgate.net
Mechanism of quinone methide formation from ferrocifen. This mechanism O-Quinolinone Quinone Methide 1) have been widely used in organic synthesis since the initial report by fries et al. This reaction is highly selective and proceeds smoothly under aqueous conditions. Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. O-Quinolinone Quinone Methide.
From pubs.acs.org
A New and Efficient Method for oQuinone Methide Intermediate O-Quinolinone Quinone Methide 1) have been widely used in organic synthesis since the initial report by fries et al. Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. This reaction is highly selective and proceeds smoothly under aqueous conditions. O-Quinolinone Quinone Methide.
From pubs.acs.org
Chemoenzymatic oQuinone Methide Formation Journal of the American O-Quinolinone Quinone Methide This reaction is highly selective and proceeds smoothly under aqueous conditions. 1) have been widely used in organic synthesis since the initial report by fries et al. Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. O-Quinolinone Quinone Methide.
From www.mdpi.com
IJMS Free FullText Reactivities of Quinone Methides versus o O-Quinolinone Quinone Methide 1) have been widely used in organic synthesis since the initial report by fries et al. This reaction is highly selective and proceeds smoothly under aqueous conditions. Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. O-Quinolinone Quinone Methide.
From www.mdpi.com
Molecules Free FullText The Emergence of Quinone Methides in O-Quinolinone Quinone Methide Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. 1) have been widely used in organic synthesis since the initial report by fries et al. This reaction is highly selective and proceeds smoothly under aqueous conditions. O-Quinolinone Quinone Methide.
From www.mdpi.com
IJMS Free FullText Reactivities of Quinone Methides versus o O-Quinolinone Quinone Methide Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. 1) have been widely used in organic synthesis since the initial report by fries et al. This reaction is highly selective and proceeds smoothly under aqueous conditions. O-Quinolinone Quinone Methide.
From pubs.rsc.org
ortho Quinone methide ( o QM) a highly reactive, ephemeral and O-Quinolinone Quinone Methide 1) have been widely used in organic synthesis since the initial report by fries et al. This reaction is highly selective and proceeds smoothly under aqueous conditions. Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. O-Quinolinone Quinone Methide.
From www.researchgate.net
Photoelectron spectra for the C 7 H 6 O isomers o quinone methide, p O-Quinolinone Quinone Methide 1) have been widely used in organic synthesis since the initial report by fries et al. This reaction is highly selective and proceeds smoothly under aqueous conditions. Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. O-Quinolinone Quinone Methide.
From www.researchgate.net
Scheme 2 The tentative pathway for the formation of oquinone 7 with O-Quinolinone Quinone Methide 1) have been widely used in organic synthesis since the initial report by fries et al. This reaction is highly selective and proceeds smoothly under aqueous conditions. Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. O-Quinolinone Quinone Methide.
From pubs.rsc.org
ortho Quinone methide ( o QM) a highly reactive, ephemeral and O-Quinolinone Quinone Methide 1) have been widely used in organic synthesis since the initial report by fries et al. This reaction is highly selective and proceeds smoothly under aqueous conditions. Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. O-Quinolinone Quinone Methide.
From www.mdpi.com
IJMS Free FullText Reactivities of Quinone Methides versus o O-Quinolinone Quinone Methide Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. 1) have been widely used in organic synthesis since the initial report by fries et al. This reaction is highly selective and proceeds smoothly under aqueous conditions. O-Quinolinone Quinone Methide.
From www.researchgate.net
Routes for the synthesis of orthoquinone methides (oQMs). Download O-Quinolinone Quinone Methide This reaction is highly selective and proceeds smoothly under aqueous conditions. Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. 1) have been widely used in organic synthesis since the initial report by fries et al. O-Quinolinone Quinone Methide.
From www.mdpi.com
IJMS Free FullText Reactivities of Quinone Methides versus o O-Quinolinone Quinone Methide 1) have been widely used in organic synthesis since the initial report by fries et al. This reaction is highly selective and proceeds smoothly under aqueous conditions. Quinone methides (qms) are transient reactive species that can be efficiently generated from stable precursors under a. O-Quinolinone Quinone Methide.