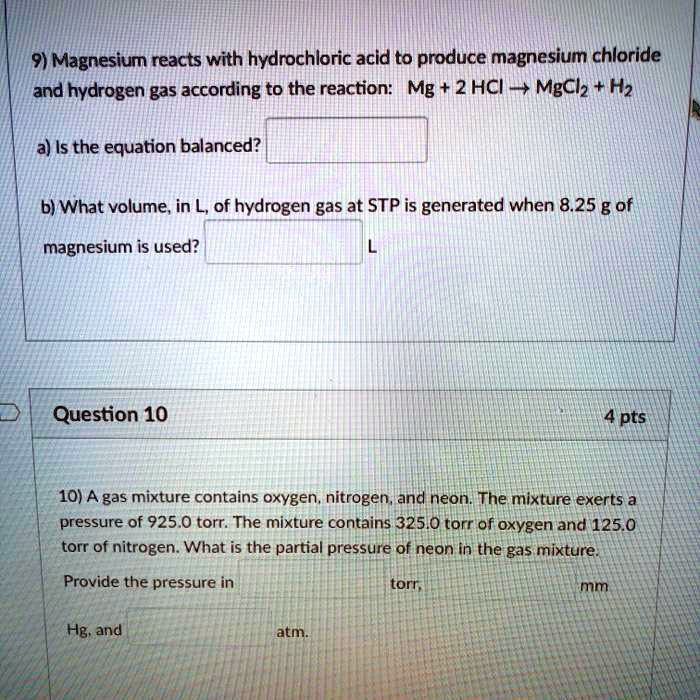
Magnesium Chloride And Hydrogen Gas . the reaction between magnesium and hydrochloric acid combine to form a salt of magnesium chloride and. Mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g). Magnesium + hydrochloric acid →. Mg (s) + 2 hcl (aq) → mgcl 2. The magnesium dissolves to form magnesium chloride,. magnesium reacts with hydrochloric acid, producing hydrogen gas according to equation 1. Magnesium is a very reactive metal. the equation for the reaction is: magnesium and dilute hydrochloric acid react together according to the equation below: magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. Magnesium + hydrochloric acid → magnesium chloride + hydrogen. magnesium chloride and hydrogen gas. adding magnesium metal to hydrochloric acid produces hydrogen gas. Acids react with metals to form a metal salt and hydrogen gas.
from www.numerade.com
magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. Magnesium is a very reactive metal. Mg (s) + 2 hcl (aq) → mgcl 2. adding magnesium metal to hydrochloric acid produces hydrogen gas. magnesium and dilute hydrochloric acid react together according to the equation below: magnesium chloride and hydrogen gas. magnesium reacts with hydrochloric acid, producing hydrogen gas according to equation 1. the equation for the reaction is: Acids react with metals to form a metal salt and hydrogen gas. the reaction between magnesium and hydrochloric acid combine to form a salt of magnesium chloride and.
SOLVED 9) Magnesium reacts with hydrochloric acid to produce magnesium
Magnesium Chloride And Hydrogen Gas magnesium and dilute hydrochloric acid react together according to the equation below: magnesium reacts with hydrochloric acid, producing hydrogen gas according to equation 1. Mg (s) + 2 hcl (aq) → mgcl 2. The magnesium dissolves to form magnesium chloride,. Mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g). Acids react with metals to form a metal salt and hydrogen gas. Magnesium + hydrochloric acid →. adding magnesium metal to hydrochloric acid produces hydrogen gas. the reaction between magnesium and hydrochloric acid combine to form a salt of magnesium chloride and. Magnesium is a very reactive metal. Magnesium + hydrochloric acid → magnesium chloride + hydrogen. magnesium and dilute hydrochloric acid react together according to the equation below: magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. magnesium chloride and hydrogen gas. the equation for the reaction is:
From www.elevise.co.uk
C2 A) Ionic Bonds AQA Combined Science Trilogy Elevise Magnesium Chloride And Hydrogen Gas Mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g). The magnesium dissolves to form magnesium chloride,. Magnesium + hydrochloric acid → magnesium chloride + hydrogen. the equation for the reaction is: Mg (s) + 2 hcl (aq) → mgcl 2. the reaction between magnesium and hydrochloric acid combine to form a salt of magnesium chloride and.. Magnesium Chloride And Hydrogen Gas.
From www.numerade.com
SOLVED 9) Magnesium reacts with hydrochloric acid to produce magnesium Magnesium Chloride And Hydrogen Gas Mg (s) + 2 hcl (aq) → mgcl 2. Magnesium + hydrochloric acid →. Magnesium is a very reactive metal. Mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g). Acids react with metals to form a metal salt and hydrogen gas. The magnesium dissolves to form magnesium chloride,. adding magnesium metal to hydrochloric acid produces hydrogen gas.. Magnesium Chloride And Hydrogen Gas.
From www.thesciencehive.co.uk
Mass and Mole Calculations (AQA) — the science sauce Magnesium Chloride And Hydrogen Gas magnesium and dilute hydrochloric acid react together according to the equation below: Mg (s) + 2 hcl (aq) → mgcl 2. Mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g). The magnesium dissolves to form magnesium chloride,. Magnesium is a very reactive metal. magnesium chloride and hydrogen gas. magnesium reacts with hydrochloric acid, producing hydrogen. Magnesium Chloride And Hydrogen Gas.
From www.coursehero.com
[Solved] 5. when solid magnesium reacts with aqueous hydrochloric acid Magnesium Chloride And Hydrogen Gas Magnesium + hydrochloric acid → magnesium chloride + hydrogen. magnesium chloride and hydrogen gas. adding magnesium metal to hydrochloric acid produces hydrogen gas. Acids react with metals to form a metal salt and hydrogen gas. the reaction between magnesium and hydrochloric acid combine to form a salt of magnesium chloride and. magnesium chloride (mgcl₂) is an. Magnesium Chloride And Hydrogen Gas.
From mammothmemory.net
Similarly magnesium and hydrochloric acid is a slow reaction Magnesium Chloride And Hydrogen Gas Magnesium + hydrochloric acid →. Magnesium + hydrochloric acid → magnesium chloride + hydrogen. magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. Mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g). The magnesium dissolves to form magnesium chloride,. the equation for the reaction is: Magnesium is a very reactive. Magnesium Chloride And Hydrogen Gas.
From www.chegg.com
Solved Magnesium metal reacts with hydrogen chloride gas to Magnesium Chloride And Hydrogen Gas Magnesium + hydrochloric acid →. magnesium chloride and hydrogen gas. magnesium and dilute hydrochloric acid react together according to the equation below: magnesium reacts with hydrochloric acid, producing hydrogen gas according to equation 1. Mg (s) + 2 hcl (aq) → mgcl 2. Acids react with metals to form a metal salt and hydrogen gas. Mg(s) +. Magnesium Chloride And Hydrogen Gas.
From www.numerade.com
SOLVED In a laboratory investigation, magnesium reacts with Magnesium Chloride And Hydrogen Gas adding magnesium metal to hydrochloric acid produces hydrogen gas. Acids react with metals to form a metal salt and hydrogen gas. the equation for the reaction is: magnesium and dilute hydrochloric acid react together according to the equation below: magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. The magnesium. Magnesium Chloride And Hydrogen Gas.
From www.numerade.com
SOLVED Magnesium reacts with hydrochloric acid to produce magnesium Magnesium Chloride And Hydrogen Gas the reaction between magnesium and hydrochloric acid combine to form a salt of magnesium chloride and. Mg (s) + 2 hcl (aq) → mgcl 2. magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. Magnesium is a very reactive metal. the equation for the reaction is: Acids react with metals to. Magnesium Chloride And Hydrogen Gas.
From www.numerade.com
SOLVED Magnesium metal reacts with hydrogen chloride gas to form Magnesium Chloride And Hydrogen Gas Mg (s) + 2 hcl (aq) → mgcl 2. magnesium and dilute hydrochloric acid react together according to the equation below: magnesium reacts with hydrochloric acid, producing hydrogen gas according to equation 1. Magnesium + hydrochloric acid → magnesium chloride + hydrogen. magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions.. Magnesium Chloride And Hydrogen Gas.
From www.numerade.com
SOLVED Magnesium metal reacts with hydrochloric acid to produce Magnesium Chloride And Hydrogen Gas Magnesium + hydrochloric acid →. Magnesium is a very reactive metal. magnesium chloride and hydrogen gas. magnesium reacts with hydrochloric acid, producing hydrogen gas according to equation 1. The magnesium dissolves to form magnesium chloride,. magnesium and dilute hydrochloric acid react together according to the equation below: Mg (s) + 2 hcl (aq) → mgcl 2. . Magnesium Chloride And Hydrogen Gas.
From www.slideserve.com
PPT Balancing Chemical Reactions PowerPoint Presentation, free Magnesium Chloride And Hydrogen Gas The magnesium dissolves to form magnesium chloride,. Magnesium + hydrochloric acid →. adding magnesium metal to hydrochloric acid produces hydrogen gas. the reaction between magnesium and hydrochloric acid combine to form a salt of magnesium chloride and. magnesium and dilute hydrochloric acid react together according to the equation below: magnesium chloride (mgcl₂) is an ionic compound. Magnesium Chloride And Hydrogen Gas.
From www.numerade.com
SOLVED When hydrochloric acid reacts with magnesium metal, hydrogen Magnesium Chloride And Hydrogen Gas magnesium chloride and hydrogen gas. Magnesium + hydrochloric acid →. magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. adding magnesium metal to hydrochloric acid produces hydrogen gas. Mg (s) + 2 hcl (aq) → mgcl 2. The magnesium dissolves to form magnesium chloride,. magnesium and dilute hydrochloric acid react. Magnesium Chloride And Hydrogen Gas.
From www.bartleby.com
Answered Magnesium metal reacts with… bartleby Magnesium Chloride And Hydrogen Gas Acids react with metals to form a metal salt and hydrogen gas. magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. Magnesium + hydrochloric acid → magnesium chloride + hydrogen. adding magnesium metal to hydrochloric acid produces hydrogen gas. magnesium and dilute hydrochloric acid react together according to the equation below:. Magnesium Chloride And Hydrogen Gas.
From www.numerade.com
SOLVED (a) Write the balanced chemical equation following reaction. 1 Magnesium Chloride And Hydrogen Gas the reaction between magnesium and hydrochloric acid combine to form a salt of magnesium chloride and. Mg (s) + 2 hcl (aq) → mgcl 2. magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. magnesium and dilute hydrochloric acid react together according to the equation below: Magnesium + hydrochloric acid →.. Magnesium Chloride And Hydrogen Gas.
From www.nagwa.com
Question Video Identifying the Name of the Gas Produced When Magnesium Magnesium Chloride And Hydrogen Gas adding magnesium metal to hydrochloric acid produces hydrogen gas. the equation for the reaction is: Magnesium + hydrochloric acid →. magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. magnesium reacts with hydrochloric acid, producing hydrogen gas according to equation 1. magnesium and dilute hydrochloric acid react together according. Magnesium Chloride And Hydrogen Gas.
From www.youtube.com
Magnesium + Hydrochloric Acid Reaction 💥 Bonus Hydrogen Explosion Magnesium Chloride And Hydrogen Gas Acids react with metals to form a metal salt and hydrogen gas. the reaction between magnesium and hydrochloric acid combine to form a salt of magnesium chloride and. Mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g). magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. magnesium chloride and. Magnesium Chloride And Hydrogen Gas.
From www.numerade.com
SOLVED Magnesium reacts with hydrochloric acid to produce magnesium Magnesium Chloride And Hydrogen Gas magnesium chloride and hydrogen gas. magnesium reacts with hydrochloric acid, producing hydrogen gas according to equation 1. adding magnesium metal to hydrochloric acid produces hydrogen gas. The magnesium dissolves to form magnesium chloride,. the reaction between magnesium and hydrochloric acid combine to form a salt of magnesium chloride and. Magnesium + hydrochloric acid →. Acids react. Magnesium Chloride And Hydrogen Gas.
From slideplayer.com
Unit 7 Chemical Reactions Conservation of Mass ppt download Magnesium Chloride And Hydrogen Gas the equation for the reaction is: magnesium chloride and hydrogen gas. Mg (s) + 2 hcl (aq) → mgcl 2. the reaction between magnesium and hydrochloric acid combine to form a salt of magnesium chloride and. Magnesium + hydrochloric acid → magnesium chloride + hydrogen. The magnesium dissolves to form magnesium chloride,. Acids react with metals to. Magnesium Chloride And Hydrogen Gas.
From www.numerade.com
SOLVED Calculate ΔHrxn for the reaction between magnesium metal and Magnesium Chloride And Hydrogen Gas Mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g). adding magnesium metal to hydrochloric acid produces hydrogen gas. the equation for the reaction is: The magnesium dissolves to form magnesium chloride,. magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. Magnesium + hydrochloric acid → magnesium chloride + hydrogen.. Magnesium Chloride And Hydrogen Gas.
From www.numerade.com
SOLVED 'Question 7 0 / 1 pts Calculate Hrxn for the reaction between Magnesium Chloride And Hydrogen Gas magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. the equation for the reaction is: Mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g). Magnesium + hydrochloric acid →. The magnesium dissolves to form magnesium chloride,. Magnesium is a very reactive metal. the reaction between magnesium and hydrochloric acid. Magnesium Chloride And Hydrogen Gas.
From www.numerade.com
SOLVED Magnesium metal reacts with hydrogen chloride gas to form Magnesium Chloride And Hydrogen Gas The magnesium dissolves to form magnesium chloride,. adding magnesium metal to hydrochloric acid produces hydrogen gas. Acids react with metals to form a metal salt and hydrogen gas. the equation for the reaction is: magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. magnesium chloride and hydrogen gas. Mg(s) +. Magnesium Chloride And Hydrogen Gas.
From www.numerade.com
Zinc and magnesium metal each reacts with hydrochloric acid to make Magnesium Chloride And Hydrogen Gas Magnesium + hydrochloric acid → magnesium chloride + hydrogen. the reaction between magnesium and hydrochloric acid combine to form a salt of magnesium chloride and. magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. magnesium and dilute hydrochloric acid react together according to the equation below: Mg (s) + 2 hcl. Magnesium Chloride And Hydrogen Gas.
From dxotceedv.blob.core.windows.net
Hydrochloric Acid To Produce Magnesium Chloride And Hydrogen Gas at Magnesium Chloride And Hydrogen Gas Mg (s) + 2 hcl (aq) → mgcl 2. The magnesium dissolves to form magnesium chloride,. the equation for the reaction is: magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. Magnesium + hydrochloric acid →. magnesium chloride and hydrogen gas. adding magnesium metal to hydrochloric acid produces hydrogen gas.. Magnesium Chloride And Hydrogen Gas.
From www.youtube.com
How to Balance Mg + HCl → MgCl2 + H2 (Magnesium + Hydrochloric Acid Magnesium Chloride And Hydrogen Gas magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. magnesium chloride and hydrogen gas. Magnesium + hydrochloric acid →. The magnesium dissolves to form magnesium chloride,. Acids react with metals to form a metal salt and hydrogen gas. Magnesium + hydrochloric acid → magnesium chloride + hydrogen. magnesium reacts with hydrochloric. Magnesium Chloride And Hydrogen Gas.
From www.numerade.com
SOLVED Solid magnesium reacts with hydrochloric acid (HCI) to form Magnesium Chloride And Hydrogen Gas magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. magnesium and dilute hydrochloric acid react together according to the equation below: The magnesium dissolves to form magnesium chloride,. the equation for the reaction is: Mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g). Magnesium + hydrochloric acid → magnesium. Magnesium Chloride And Hydrogen Gas.
From www.slideserve.com
PPT Reaction Video PowerPoint Presentation, free download ID2461229 Magnesium Chloride And Hydrogen Gas adding magnesium metal to hydrochloric acid produces hydrogen gas. Acids react with metals to form a metal salt and hydrogen gas. Mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g). The magnesium dissolves to form magnesium chloride,. the equation for the reaction is: Mg (s) + 2 hcl (aq) → mgcl 2. Magnesium + hydrochloric acid. Magnesium Chloride And Hydrogen Gas.
From omniscience.tech
Mastering Magnesium and Hydrochloric Acid Reactions Unraveling the Magnesium Chloride And Hydrogen Gas the equation for the reaction is: Mg (s) + 2 hcl (aq) → mgcl 2. magnesium and dilute hydrochloric acid react together according to the equation below: magnesium chloride and hydrogen gas. Magnesium + hydrochloric acid →. adding magnesium metal to hydrochloric acid produces hydrogen gas. magnesium reacts with hydrochloric acid, producing hydrogen gas according. Magnesium Chloride And Hydrogen Gas.
From www.numerade.com
SOLVED When 0.20g of magnesium ribbon was reacted with an excess of Magnesium Chloride And Hydrogen Gas magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. magnesium and dilute hydrochloric acid react together according to the equation below: Magnesium is a very reactive metal. Magnesium + hydrochloric acid → magnesium chloride + hydrogen. the reaction between magnesium and hydrochloric acid combine to form a salt of magnesium chloride. Magnesium Chloride And Hydrogen Gas.
From www.numerade.com
SOLVED Magnesium reacts with hydrochloric acid, HCl, to form magnesium Magnesium Chloride And Hydrogen Gas Mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g). the reaction between magnesium and hydrochloric acid combine to form a salt of magnesium chloride and. the equation for the reaction is: magnesium reacts with hydrochloric acid, producing hydrogen gas according to equation 1. Magnesium + hydrochloric acid → magnesium chloride + hydrogen. The magnesium dissolves. Magnesium Chloride And Hydrogen Gas.
From www.numerade.com
SOLVED 1. Magnesium and hydrochloric acid react to form magnesium Magnesium Chloride And Hydrogen Gas Magnesium is a very reactive metal. adding magnesium metal to hydrochloric acid produces hydrogen gas. magnesium reacts with hydrochloric acid, producing hydrogen gas according to equation 1. Magnesium + hydrochloric acid → magnesium chloride + hydrogen. magnesium chloride and hydrogen gas. the equation for the reaction is: The magnesium dissolves to form magnesium chloride,. Acids react. Magnesium Chloride And Hydrogen Gas.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Magnesium Chloride And Hydrogen Gas Magnesium + hydrochloric acid → magnesium chloride + hydrogen. Mg (s) + 2 hcl (aq) → mgcl 2. Magnesium is a very reactive metal. magnesium and dilute hydrochloric acid react together according to the equation below: magnesium chloride and hydrogen gas. the equation for the reaction is: the reaction between magnesium and hydrochloric acid combine to. Magnesium Chloride And Hydrogen Gas.
From www.chegg.com
Solved 7. (10 points) Magnesium metal reacts with Magnesium Chloride And Hydrogen Gas the reaction between magnesium and hydrochloric acid combine to form a salt of magnesium chloride and. the equation for the reaction is: adding magnesium metal to hydrochloric acid produces hydrogen gas. Acids react with metals to form a metal salt and hydrogen gas. magnesium reacts with hydrochloric acid, producing hydrogen gas according to equation 1. Mg. Magnesium Chloride And Hydrogen Gas.
From www.chegg.com
Solved 7a. The Reaction Between Magnesium Metal And Hydro... Magnesium Chloride And Hydrogen Gas the equation for the reaction is: The magnesium dissolves to form magnesium chloride,. Acids react with metals to form a metal salt and hydrogen gas. Magnesium + hydrochloric acid → magnesium chloride + hydrogen. magnesium reacts with hydrochloric acid, producing hydrogen gas according to equation 1. magnesium and dilute hydrochloric acid react together according to the equation. Magnesium Chloride And Hydrogen Gas.
From www.slideserve.com
PPT Rate of Reactions PowerPoint Presentation, free download ID508328 Magnesium Chloride And Hydrogen Gas magnesium chloride (mgcl₂) is an ionic compound formed by magnesium (mg²⁺) and chlorine (cl⁻) ions. Mg (s) + 2 hcl (aq) → mgcl 2. adding magnesium metal to hydrochloric acid produces hydrogen gas. Magnesium + hydrochloric acid → magnesium chloride + hydrogen. magnesium reacts with hydrochloric acid, producing hydrogen gas according to equation 1. magnesium and. Magnesium Chloride And Hydrogen Gas.
From www.numerade.com
SOLVED When 0.20g of magnesium ribbon was reacted with an excess of Magnesium Chloride And Hydrogen Gas Magnesium + hydrochloric acid → magnesium chloride + hydrogen. Magnesium is a very reactive metal. Mg(s) + 2hcl(aq) → mgcl 2 (aq) + h 2 (g). magnesium reacts with hydrochloric acid, producing hydrogen gas according to equation 1. magnesium and dilute hydrochloric acid react together according to the equation below: Mg (s) + 2 hcl (aq) → mgcl. Magnesium Chloride And Hydrogen Gas.