Calorimeter Definition Simple . Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. It mainly consists of a metallic vessel made of materials which are good. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. A calorimeter is a device used for heat measurements necessary for calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. The process of measuring this heat is called calorimetry. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. A calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction.
from www.scienceabc.com
A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. For example, when an exothermic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is a device used for heat measurements necessary for calorimetry. The process of measuring this heat is called calorimetry. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. A calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction. It mainly consists of a metallic vessel made of materials which are good.
Molar Heat Capacity Definition, Formula, Equation, Calculation
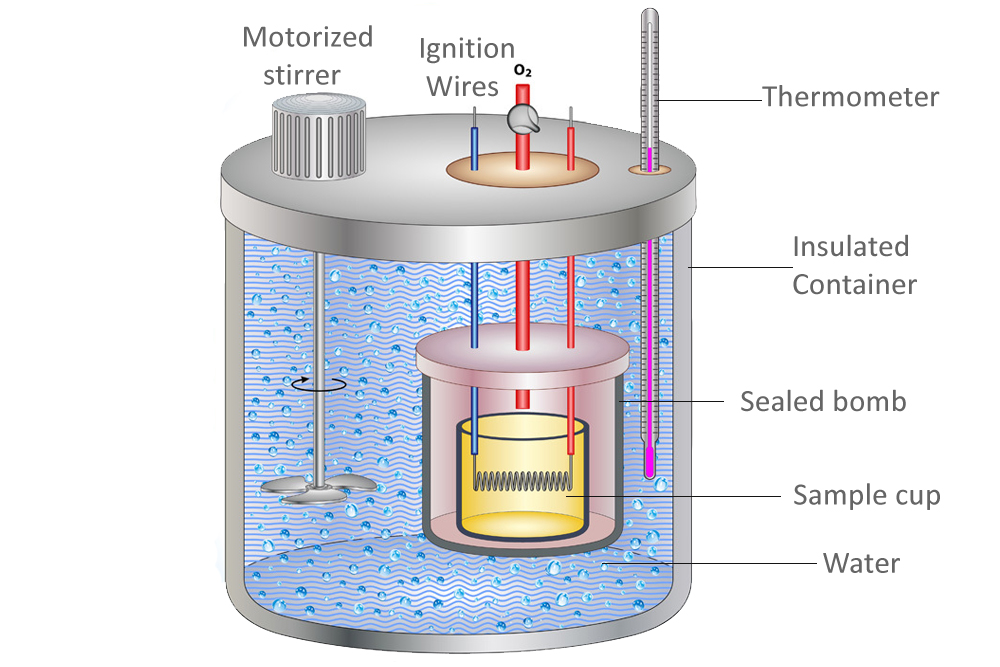
Calorimeter Definition Simple A calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction. It mainly consists of a metallic vessel made of materials which are good. For example, when an exothermic. A calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. The process of measuring this heat is called calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used for heat measurements necessary for calorimetry.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Calorimeter Definition Simple Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is a device used for heat measurements necessary for calorimetry. A calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction. The process of measuring this heat is. Calorimeter Definition Simple.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Calorimeter Definition Simple The process of measuring this heat is called calorimetry. For example, when an exothermic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. A calorimeter is a. Calorimeter Definition Simple.
From animalia-life.club
Simple Bomb Calorimeter Calorimeter Definition Simple For example, when an exothermic. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. A calorimeter is a device used for heat measurements necessary for calorimetry. The process of measuring this heat is called calorimetry. It mainly consists of a metallic vessel made of materials which are. Calorimeter Definition Simple.
From www.pinterest.com
Calorimetry Bomb Calorimeter Experiment Science fair, Homeschool and Calorimeter Definition Simple A calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is a device used for heat measurements necessary for calorimetry. Calorimetry is a branch of science concerned. Calorimeter Definition Simple.
From chemwiki.ucdavis.edu
Chapter 9.6 Calorimetry Chemwiki Calorimeter Definition Simple A calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. A calorimeter is a device used for heat measurements necessary for calorimetry. It mainly consists of a metallic vessel made of. Calorimeter Definition Simple.
From loevclgsu.blob.core.windows.net
Calorimeter Thermochemistry Definition at Leona Fraher blog Calorimeter Definition Simple The process of measuring this heat is called calorimetry. A calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction. A calorimeter is a device used for heat measurements necessary for calorimetry. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. Calorimeter Definition Simple.
From exollkmhz.blob.core.windows.net
Define Adiabatic Calorimeter at Alma Martin blog Calorimeter Definition Simple Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. The process of measuring this heat is called calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A calorimeter is a device used to. Calorimeter Definition Simple.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimeter Definition Simple A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. For. Calorimeter Definition Simple.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Calorimeter Definition Simple The process of measuring this heat is called calorimetry. For example, when an exothermic. A calorimeter is a device used for heat measurements necessary for calorimetry. A calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction. It mainly consists of a metallic vessel made of materials which are good.. Calorimeter Definition Simple.
From quizlet.com
Sketch a simple calorimeter and label the purpose of each co Quizlet Calorimeter Definition Simple Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change.. Calorimeter Definition Simple.
From martinfersbanks.blogspot.com
Is a Bomb Calorimeter Constant Pressure Calorimeter Definition Simple A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction. It. Calorimeter Definition Simple.
From exollkmhz.blob.core.windows.net
Define Adiabatic Calorimeter at Alma Martin blog Calorimeter Definition Simple A calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is a device used for heat measurements necessary for calorimetry. A calorimeter is a device used to. Calorimeter Definition Simple.
From ar.inspiredpencil.com
Simple Bomb Calorimeter Calorimeter Definition Simple It mainly consists of a metallic vessel made of materials which are good. For example, when an exothermic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is. Calorimeter Definition Simple.
From www.slideserve.com
PPT a “ Calorimeter ” PowerPoint Presentation, free download ID7050684 Calorimeter Definition Simple Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. A calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical. Calorimeter Definition Simple.
From ar.inspiredpencil.com
Simple Bomb Calorimeter Calorimeter Definition Simple A calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It mainly consists of a metallic vessel made of materials which are good. A calorimeter is a device used for. Calorimeter Definition Simple.
From www.youtube.com
Bomb Calorimeter Definition, Construction, Working & Uses YouTube Calorimeter Definition Simple Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. For example, when an exothermic. A calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction. A calorimeter is a device used to measure the heat flow of. Calorimeter Definition Simple.
From courses.lumenlearning.com
Calorimetry Chemistry I Calorimeter Definition Simple Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. A calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating. Calorimeter Definition Simple.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Calorimeter Definition Simple A calorimeter is a device used for heat measurements necessary for calorimetry. For example, when an exothermic. A calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. Calorimetry is a branch. Calorimeter Definition Simple.
From exonwzknq.blob.core.windows.net
Calorimetry Experiment Gcse Chemistry at Sanders blog Calorimeter Definition Simple Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. For example, when an exothermic. A calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction. A calorimeter is a device used to measure the amount of heat. Calorimeter Definition Simple.
From gamesmartz.com
Calorimeter Definition Easy to Understand Calorimeter Definition Simple A calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction. It mainly consists of a metallic vessel made of materials which are good. The process of measuring this heat is called calorimetry. A calorimeter is a device used for heat measurements necessary for calorimetry. Calorimeter, device for measuring the. Calorimeter Definition Simple.
From glossary.periodni.com
Download calorimeter.png image from Calorimeter Definition Simple Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. For example, when an exothermic. A calorimeter is a device used for heat measurements necessary for calorimetry. The process of measuring this heat is called calorimetry. A calorimeter is a device used to measure the heat flow of. Calorimeter Definition Simple.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimeter Definition Simple Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. The process of measuring this heat is called calorimetry. A calorimeter is a device used for heat measurements necessary for calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or. Calorimeter Definition Simple.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Calorimeter Definition Simple Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. The process of measuring this heat is called calorimetry. It mainly consists of a metallic vessel made of materials which are good. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change.. Calorimeter Definition Simple.
From www.tessshebaylo.com
Equation For Determining Calorimetry Tessshebaylo Calorimeter Definition Simple Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The process of measuring this heat is called calorimetry. A calorimeter is a tool that measures the amount of thermal energy. Calorimeter Definition Simple.
From ar.inspiredpencil.com
Calorimeter Diagram Calorimeter Definition Simple The process of measuring this heat is called calorimetry. It mainly consists of a metallic vessel made of materials which are good. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. Calorimeter Definition Simple.
From www.savemyexams.com
Calorimetry Experiments SL IB Chemistry Revision Notes 2025 Save My Calorimeter Definition Simple For example, when an exothermic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter is a device used for heat measurements necessary for calorimetry. The process of measuring this heat is called calorimetry. It mainly consists of a metallic vessel made of materials which are good. A. Calorimeter Definition Simple.
From www.youtube.com
Principle of Calorimetry YouTube Calorimeter Definition Simple Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. A calorimeter is a device used for heat measurements necessary for calorimetry. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. It mainly consists of a metallic vessel made. Calorimeter Definition Simple.
From loevclgsu.blob.core.windows.net
Calorimeter Thermochemistry Definition at Leona Fraher blog Calorimeter Definition Simple A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. Calorimeter Definition Simple.
From ar.inspiredpencil.com
Calorimeter Diagram Calorimeter Definition Simple Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. A calorimeter is a device used for heat measurements necessary for calorimetry. A calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction. For example, when an exothermic.. Calorimeter Definition Simple.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimeter Definition Simple It mainly consists of a metallic vessel made of materials which are good. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. The process of measuring this heat is called calorimetry. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a. Calorimeter Definition Simple.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimeter Definition Simple A calorimeter is a device used for heat measurements necessary for calorimetry. For example, when an exothermic. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. A calorimeter is. Calorimeter Definition Simple.
From scienceinfo.com
Calorimeter Definition, Types and Uses Calorimeter Definition Simple Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. A calorimeter is a device used for heat measurements necessary for calorimetry. The process of measuring this heat is called calorimetry. A calorimeter is a tool that measures the amount of thermal energy contained in a substance, or. Calorimeter Definition Simple.
From loerzgmcm.blob.core.windows.net
How To Make A Homemade Calorimeter at Norma Mercer blog Calorimeter Definition Simple A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. A calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in. Calorimeter Definition Simple.
From exourbxre.blob.core.windows.net
Why Is Calorimeter Constant Important at Waldman blog Calorimeter Definition Simple Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. A calorimeter is a device used for heat measurements necessary for calorimetry. For example, when an exothermic. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A. Calorimeter Definition Simple.
From ar.inspiredpencil.com
Simple Bomb Calorimeter Calorimeter Definition Simple A calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. A calorimeter is a tool that measures the amount of thermal energy contained in a substance, or released in a reaction. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is. Calorimeter Definition Simple.