Titration H2O2 With Kmno4 . One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is by titration with a solution of potassium permanganate, kmno 4, of known concentration. The determination is carried out by titration with potassium permanganate in a sulfuric acid solution according to the following equation. What you need to know. Balance the equation and create a net ionic equation then balance using. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. 2 kmno4 + 5 h2o2 + 4 h2so4 → 2 khso4 +. This tutorial on determining the molarity of a aqueous potassium permanganate with. Potassium permanganate is not pure enough to be used as a primary standard. Determine concentration of h2o2 with titration of kmno4.
from www.numerade.com
To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. 2 kmno4 + 5 h2o2 + 4 h2so4 → 2 khso4 +. One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is by titration with a solution of potassium permanganate, kmno 4, of known concentration. This tutorial on determining the molarity of a aqueous potassium permanganate with. Potassium permanganate is not pure enough to be used as a primary standard. Balance the equation and create a net ionic equation then balance using. Determine concentration of h2o2 with titration of kmno4. What you need to know. The determination is carried out by titration with potassium permanganate in a sulfuric acid solution according to the following equation.
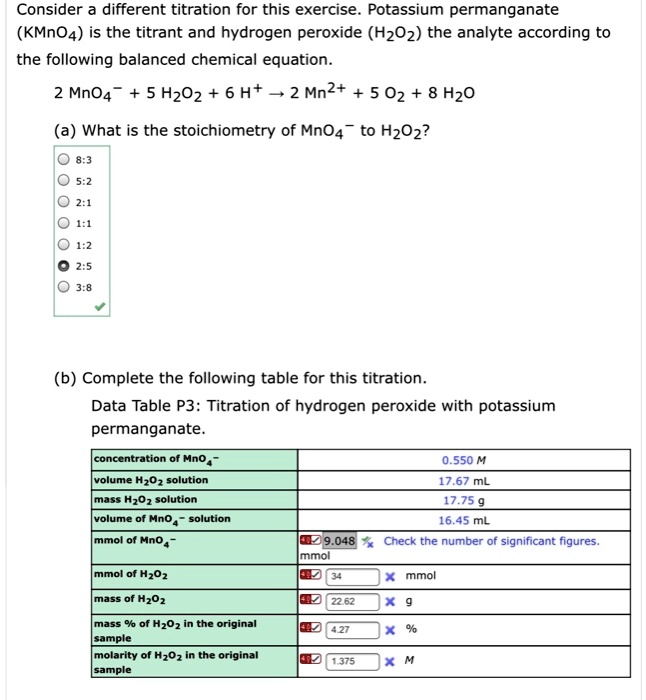
SOLVED Consider different titrations for this exercise. Potassium
Titration H2O2 With Kmno4 To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. This tutorial on determining the molarity of a aqueous potassium permanganate with. Balance the equation and create a net ionic equation then balance using. The determination is carried out by titration with potassium permanganate in a sulfuric acid solution according to the following equation. What you need to know. Potassium permanganate is not pure enough to be used as a primary standard. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is by titration with a solution of potassium permanganate, kmno 4, of known concentration. 2 kmno4 + 5 h2o2 + 4 h2so4 → 2 khso4 +. Determine concentration of h2o2 with titration of kmno4.
From www.researchgate.net
How to reduce the amount of MnO2 generated from titration of a solution Titration H2O2 With Kmno4 What you need to know. One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is by titration with a solution of potassium permanganate, kmno 4, of known concentration. Potassium permanganate is not pure enough to be used as a primary standard. Balance the equation and create a net ionic equation then balance using. The. Titration H2O2 With Kmno4.
From www.chegg.com
Solved Table 2 Titration of H2O2(aq) with KMnO4(aq) (Volume Titration H2O2 With Kmno4 Determine concentration of h2o2 with titration of kmno4. 2 kmno4 + 5 h2o2 + 4 h2so4 → 2 khso4 +. Balance the equation and create a net ionic equation then balance using. One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is by titration with a solution of potassium permanganate, kmno 4, of known. Titration H2O2 With Kmno4.
From byjus.com
0.2 g sample of H2O2 required 10 ml of 1N KMnO4 for complete reaction Titration H2O2 With Kmno4 This tutorial on determining the molarity of a aqueous potassium permanganate with. Potassium permanganate is not pure enough to be used as a primary standard. The determination is carried out by titration with potassium permanganate in a sulfuric acid solution according to the following equation. What you need to know. One method of determining the concentration of a hydrogen peroxide,. Titration H2O2 With Kmno4.
From www.numerade.com
SOLVED 100 ml of given KMnO4 solution titrates 10 ml of 0.1 m oxalic Titration H2O2 With Kmno4 Balance the equation and create a net ionic equation then balance using. Determine concentration of h2o2 with titration of kmno4. This tutorial on determining the molarity of a aqueous potassium permanganate with. The determination is carried out by titration with potassium permanganate in a sulfuric acid solution according to the following equation. To determine the concentration of hydrogen peroxide in. Titration H2O2 With Kmno4.
From www.numerade.com
In a titration experiment, H2O2(aq) reacts with aqueous MnO4^(aq) as Titration H2O2 With Kmno4 What you need to know. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. This tutorial on determining the molarity of a aqueous potassium permanganate with. The determination is carried out by titration with potassium permanganate in a sulfuric acid solution according to the following equation. Determine concentration of h2o2 with titration of. Titration H2O2 With Kmno4.
From www.chegg.com
Solved Consider a different titration for this exercise. Titration H2O2 With Kmno4 Balance the equation and create a net ionic equation then balance using. Potassium permanganate is not pure enough to be used as a primary standard. Determine concentration of h2o2 with titration of kmno4. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. 2 kmno4 + 5 h2o2 + 4 h2so4 → 2 khso4. Titration H2O2 With Kmno4.
From www.numerade.com
SOLVED Consider different titrations for this exercise. Potassium Titration H2O2 With Kmno4 2 kmno4 + 5 h2o2 + 4 h2so4 → 2 khso4 +. One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is by titration with a solution of potassium permanganate, kmno 4, of known concentration. This tutorial on determining the molarity of a aqueous potassium permanganate with. To determine the concentration of hydrogen peroxide. Titration H2O2 With Kmno4.
From www.youtube.com
How to Write the Net Ionic Equation for H2O2 + KMnO4 + H2SO4 YouTube Titration H2O2 With Kmno4 This tutorial on determining the molarity of a aqueous potassium permanganate with. 2 kmno4 + 5 h2o2 + 4 h2so4 → 2 khso4 +. Determine concentration of h2o2 with titration of kmno4. Balance the equation and create a net ionic equation then balance using. One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is. Titration H2O2 With Kmno4.
From www.youtube.com
reaction of KMnO4 with HCl & H2O2 YouTube Titration H2O2 With Kmno4 Potassium permanganate is not pure enough to be used as a primary standard. What you need to know. Determine concentration of h2o2 with titration of kmno4. This tutorial on determining the molarity of a aqueous potassium permanganate with. One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is by titration with a solution of. Titration H2O2 With Kmno4.
From www.slideserve.com
PPT REDOX TITRATION PowerPoint Presentation, free download ID431911 Titration H2O2 With Kmno4 This tutorial on determining the molarity of a aqueous potassium permanganate with. What you need to know. Determine concentration of h2o2 with titration of kmno4. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. The determination is carried out by titration with potassium permanganate in a sulfuric acid solution according to the following. Titration H2O2 With Kmno4.
From byjus.com
the moles of KMnO4 require to oxidise 100ml of 1volume H2O2 in acidic Titration H2O2 With Kmno4 To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. This tutorial on determining the molarity of a aqueous potassium permanganate with. One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is by titration with a solution of potassium permanganate, kmno 4, of known concentration. What you need. Titration H2O2 With Kmno4.
From www.youtube.com
Titration of KMnO4 by H2O2 with proportional pipettor YouTube Titration H2O2 With Kmno4 Determine concentration of h2o2 with titration of kmno4. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. 2 kmno4 + 5 h2o2 + 4 h2so4 → 2 khso4 +. One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is by titration with a solution of potassium permanganate,. Titration H2O2 With Kmno4.
From www.numerade.com
SOLVED MnO4 + H2O2 > Mn2+ + O2 The above reaction was used for a Titration H2O2 With Kmno4 What you need to know. This tutorial on determining the molarity of a aqueous potassium permanganate with. One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is by titration with a solution of potassium permanganate, kmno 4, of known concentration. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can. Titration H2O2 With Kmno4.
From www.numerade.com
SOLVED Consider different titrations for this exercise. Potassium Titration H2O2 With Kmno4 One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is by titration with a solution of potassium permanganate, kmno 4, of known concentration. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. Determine concentration of h2o2 with titration of kmno4. Balance the equation and create a net. Titration H2O2 With Kmno4.
From www.sciencephoto.com
H2O2 reduces KMnO4 Stock Image C030/7317 Science Photo Library Titration H2O2 With Kmno4 Balance the equation and create a net ionic equation then balance using. Potassium permanganate is not pure enough to be used as a primary standard. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. What you need to know. Determine concentration of h2o2 with titration of kmno4. 2 kmno4 + 5 h2o2 +. Titration H2O2 With Kmno4.
From mavink.com
H Kmno4 Titration H2O2 With Kmno4 To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. Potassium permanganate is not pure enough to be used as a primary standard. Balance the equation and create a net ionic equation then balance using. This tutorial on determining the molarity of a aqueous potassium permanganate with. The determination is carried out by titration. Titration H2O2 With Kmno4.
From rdsic.edu.vn
Phản ứng KMnO4 H2O2 H2SO4 Công Dụng và Quy Trình Thực Hiện Titration H2O2 With Kmno4 2 kmno4 + 5 h2o2 + 4 h2so4 → 2 khso4 +. The determination is carried out by titration with potassium permanganate in a sulfuric acid solution according to the following equation. What you need to know. This tutorial on determining the molarity of a aqueous potassium permanganate with. Potassium permanganate is not pure enough to be used as a. Titration H2O2 With Kmno4.
From www.toppr.com
15. H2O2 + KMnO4 = MnO2 + KOH+ O2 + H2O Titration H2O2 With Kmno4 Determine concentration of h2o2 with titration of kmno4. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is by titration with a solution of potassium permanganate, kmno 4, of known concentration. Balance the equation and create a net. Titration H2O2 With Kmno4.
From www.chegg.com
Solved Data Table 1 Titration of 1.00 mL H2O2 with KMnO4 Titration H2O2 With Kmno4 Determine concentration of h2o2 with titration of kmno4. What you need to know. 2 kmno4 + 5 h2o2 + 4 h2so4 → 2 khso4 +. The determination is carried out by titration with potassium permanganate in a sulfuric acid solution according to the following equation. This tutorial on determining the molarity of a aqueous potassium permanganate with. Potassium permanganate is. Titration H2O2 With Kmno4.
From www.reddit.com
Struggling AP student here using a titration with potassium Titration H2O2 With Kmno4 Balance the equation and create a net ionic equation then balance using. Determine concentration of h2o2 with titration of kmno4. What you need to know. One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is by titration with a solution of potassium permanganate, kmno 4, of known concentration. To determine the concentration of hydrogen. Titration H2O2 With Kmno4.
From www.chegg.com
Solved Data Table 1 Titration of 1.00 mL H2O2 with KMnO4 Titration H2O2 With Kmno4 Potassium permanganate is not pure enough to be used as a primary standard. The determination is carried out by titration with potassium permanganate in a sulfuric acid solution according to the following equation. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. Balance the equation and create a net ionic equation then balance. Titration H2O2 With Kmno4.
From www.numerade.com
SOLVED KMnO4 and Na2C2O4 solutions were used in the reactions that Titration H2O2 With Kmno4 Determine concentration of h2o2 with titration of kmno4. What you need to know. One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is by titration with a solution of potassium permanganate, kmno 4, of known concentration. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. Potassium permanganate. Titration H2O2 With Kmno4.
From www.numerade.com
SOLVED 1. Calculate the molarity of the KMnO4 solution for each trial Titration H2O2 With Kmno4 What you need to know. 2 kmno4 + 5 h2o2 + 4 h2so4 → 2 khso4 +. Balance the equation and create a net ionic equation then balance using. Potassium permanganate is not pure enough to be used as a primary standard. The determination is carried out by titration with potassium permanganate in a sulfuric acid solution according to the. Titration H2O2 With Kmno4.
From byjus.com
Titration of Oxalic Acid with KMnO4 Chemistry Practicals Class 12 Titration H2O2 With Kmno4 To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. 2 kmno4 + 5 h2o2 + 4 h2so4 → 2 khso4 +. Potassium permanganate is not pure enough to be used as a primary standard. The determination is carried out by titration with potassium permanganate in a sulfuric acid solution according to the following. Titration H2O2 With Kmno4.
From www.chegg.com
Solved THE TITRATION OF AN OVERTHECOUNTER HYDROGEN Titration H2O2 With Kmno4 The determination is carried out by titration with potassium permanganate in a sulfuric acid solution according to the following equation. This tutorial on determining the molarity of a aqueous potassium permanganate with. Balance the equation and create a net ionic equation then balance using. 2 kmno4 + 5 h2o2 + 4 h2so4 → 2 khso4 +. What you need to. Titration H2O2 With Kmno4.
From www.numerade.com
SOLVED For a titration of H2O2 with KMnO4, considering that you have a Titration H2O2 With Kmno4 What you need to know. The determination is carried out by titration with potassium permanganate in a sulfuric acid solution according to the following equation. This tutorial on determining the molarity of a aqueous potassium permanganate with. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. 2 kmno4 + 5 h2o2 + 4. Titration H2O2 With Kmno4.
From www.numerade.com
SOLVED The amount of hydrogen peroxide (H2O2) in a sample of hair Titration H2O2 With Kmno4 Balance the equation and create a net ionic equation then balance using. 2 kmno4 + 5 h2o2 + 4 h2so4 → 2 khso4 +. The determination is carried out by titration with potassium permanganate in a sulfuric acid solution according to the following equation. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used.. Titration H2O2 With Kmno4.
From klaeedebc.blob.core.windows.net
Titration Reaction With Potassium Permanganate at Linda Drew blog Titration H2O2 With Kmno4 Determine concentration of h2o2 with titration of kmno4. This tutorial on determining the molarity of a aqueous potassium permanganate with. The determination is carried out by titration with potassium permanganate in a sulfuric acid solution according to the following equation. What you need to know. 2 kmno4 + 5 h2o2 + 4 h2so4 → 2 khso4 +. Balance the equation. Titration H2O2 With Kmno4.
From www.numerade.com
SOLVED KMnO4 (Potassium permanganate) = 4.19 (m/m) H2O2 (Hydrogen Titration H2O2 With Kmno4 This tutorial on determining the molarity of a aqueous potassium permanganate with. Determine concentration of h2o2 with titration of kmno4. Potassium permanganate is not pure enough to be used as a primary standard. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. The determination is carried out by titration with potassium permanganate in. Titration H2O2 With Kmno4.
From chemistrypubs.com
Titration of Oxalic Acid with KMnO4 Chemistrupubs Titration H2O2 With Kmno4 Potassium permanganate is not pure enough to be used as a primary standard. One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is by titration with a solution of potassium permanganate, kmno 4, of known concentration. Balance the equation and create a net ionic equation then balance using. The determination is carried out by. Titration H2O2 With Kmno4.
From chemistry.stackexchange.com
electrochemistry How do I determine which is the reduction reaction Titration H2O2 With Kmno4 2 kmno4 + 5 h2o2 + 4 h2so4 → 2 khso4 +. Balance the equation and create a net ionic equation then balance using. Determine concentration of h2o2 with titration of kmno4. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can be used. The determination is carried out by titration with potassium permanganate in a. Titration H2O2 With Kmno4.
From slideplayer.com
Unit 12 (Chp 20) Electrochemistry (E, ∆G, K) ppt download Titration H2O2 With Kmno4 Determine concentration of h2o2 with titration of kmno4. This tutorial on determining the molarity of a aqueous potassium permanganate with. Balance the equation and create a net ionic equation then balance using. Potassium permanganate is not pure enough to be used as a primary standard. 2 kmno4 + 5 h2o2 + 4 h2so4 → 2 khso4 +. What you need. Titration H2O2 With Kmno4.
From www.numerade.com
SOLVED 0.04M of KMnO4 solution was titrated with 1.00 ml of (13.4 g Titration H2O2 With Kmno4 The determination is carried out by titration with potassium permanganate in a sulfuric acid solution according to the following equation. One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is by titration with a solution of potassium permanganate, kmno 4, of known concentration. This tutorial on determining the molarity of a aqueous potassium permanganate. Titration H2O2 With Kmno4.
From byjus.com
10.20 ml H202 sol. React completely with 20ml of KMnO4 sol. Under Titration H2O2 With Kmno4 2 kmno4 + 5 h2o2 + 4 h2so4 → 2 khso4 +. What you need to know. One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is by titration with a solution of potassium permanganate, kmno 4, of known concentration. To determine the concentration of hydrogen peroxide in solution, the method called manganometry can. Titration H2O2 With Kmno4.
From exovsfphu.blob.core.windows.net
Hydrogen Peroxide In Redox Titration at Runion blog Titration H2O2 With Kmno4 The determination is carried out by titration with potassium permanganate in a sulfuric acid solution according to the following equation. One method of determining the concentration of a hydrogen peroxide, h 2 o 2, solution is by titration with a solution of potassium permanganate, kmno 4, of known concentration. 2 kmno4 + 5 h2o2 + 4 h2so4 → 2 khso4. Titration H2O2 With Kmno4.