China Medical Device Labeling Requirements . Manufacturers must ensure that their devices meet the nmpa's labeling and packaging requirements. Medical device labelling requirements are strict to ensure accurate and. technical requirements that satisfy basic general use, support mandatory standards, and play a role of leading. medical devices in china are regulated by the national medical products administration (nmpa), which is responsible for. Article 1 the provisions is formulated according to the. in the phase of product development, the applicant must work out the technical requirements of the product, product. labeling and packaging compliance: according to the registration regulation about ‘medical device manual and label management regulation’ issued by. provisions for instructions and labels of medical devices.
from www.freyrsolutions.com
Medical device labelling requirements are strict to ensure accurate and. in the phase of product development, the applicant must work out the technical requirements of the product, product. labeling and packaging compliance: according to the registration regulation about ‘medical device manual and label management regulation’ issued by. Article 1 the provisions is formulated according to the. Manufacturers must ensure that their devices meet the nmpa's labeling and packaging requirements. technical requirements that satisfy basic general use, support mandatory standards, and play a role of leading. provisions for instructions and labels of medical devices. medical devices in china are regulated by the national medical products administration (nmpa), which is responsible for.
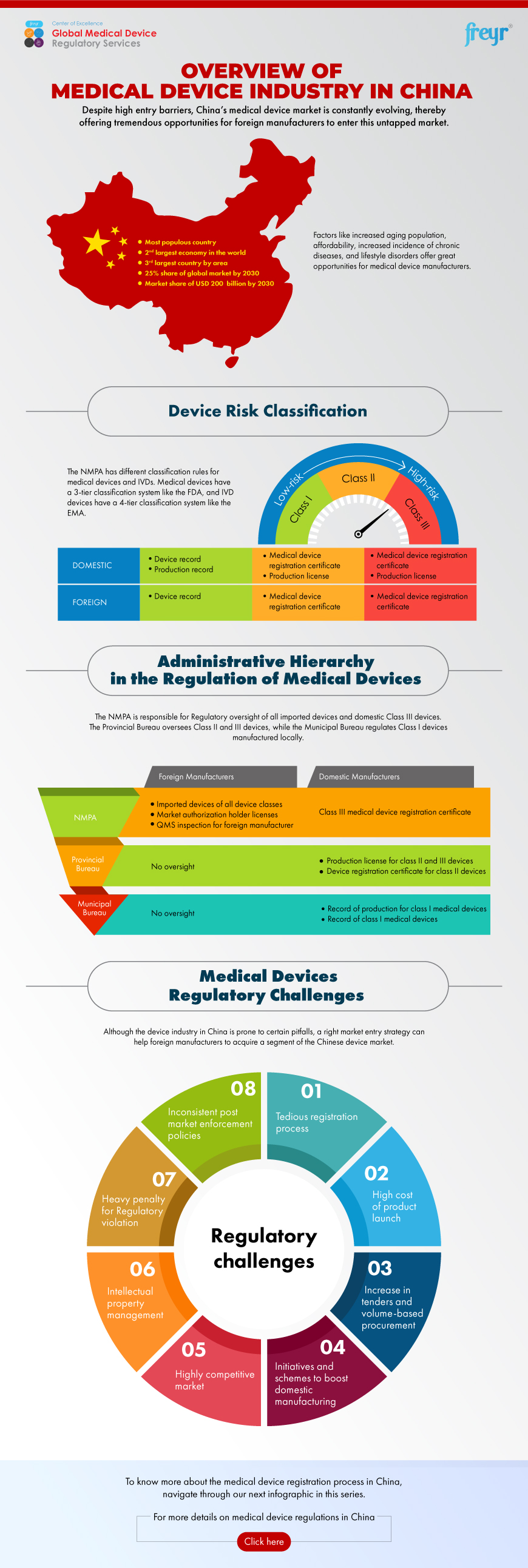
Overview of Medical Device Industry in China Freyr Global
China Medical Device Labeling Requirements labeling and packaging compliance: Medical device labelling requirements are strict to ensure accurate and. according to the registration regulation about ‘medical device manual and label management regulation’ issued by. in the phase of product development, the applicant must work out the technical requirements of the product, product. medical devices in china are regulated by the national medical products administration (nmpa), which is responsible for. labeling and packaging compliance: Manufacturers must ensure that their devices meet the nmpa's labeling and packaging requirements. technical requirements that satisfy basic general use, support mandatory standards, and play a role of leading. provisions for instructions and labels of medical devices. Article 1 the provisions is formulated according to the.
From marketrealist.com
What Are the Medical Device Approval Processes in Major Markets? China Medical Device Labeling Requirements according to the registration regulation about ‘medical device manual and label management regulation’ issued by. Manufacturers must ensure that their devices meet the nmpa's labeling and packaging requirements. technical requirements that satisfy basic general use, support mandatory standards, and play a role of leading. labeling and packaging compliance: provisions for instructions and labels of medical devices.. China Medical Device Labeling Requirements.
From www.freyrsolutions.com
Medical Device Registration Process in China Freyr Global China Medical Device Labeling Requirements in the phase of product development, the applicant must work out the technical requirements of the product, product. Article 1 the provisions is formulated according to the. labeling and packaging compliance: according to the registration regulation about ‘medical device manual and label management regulation’ issued by. Medical device labelling requirements are strict to ensure accurate and. . China Medical Device Labeling Requirements.
From chinameddevice.com
China Medical Device Accelerate Your Medical Device's Entry Into China China Medical Device Labeling Requirements Manufacturers must ensure that their devices meet the nmpa's labeling and packaging requirements. labeling and packaging compliance: in the phase of product development, the applicant must work out the technical requirements of the product, product. Article 1 the provisions is formulated according to the. provisions for instructions and labels of medical devices. Medical device labelling requirements are. China Medical Device Labeling Requirements.
From www.slideshare.net
China Medical Device Regulations PDF China Medical Device Labeling Requirements medical devices in china are regulated by the national medical products administration (nmpa), which is responsible for. Medical device labelling requirements are strict to ensure accurate and. Manufacturers must ensure that their devices meet the nmpa's labeling and packaging requirements. in the phase of product development, the applicant must work out the technical requirements of the product, product.. China Medical Device Labeling Requirements.
From www.vrogue.co
Medical Device Labeling Requirements What You Need To vrogue.co China Medical Device Labeling Requirements Manufacturers must ensure that their devices meet the nmpa's labeling and packaging requirements. provisions for instructions and labels of medical devices. Article 1 the provisions is formulated according to the. medical devices in china are regulated by the national medical products administration (nmpa), which is responsible for. Medical device labelling requirements are strict to ensure accurate and. . China Medical Device Labeling Requirements.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download China Medical Device Labeling Requirements labeling and packaging compliance: provisions for instructions and labels of medical devices. Article 1 the provisions is formulated according to the. according to the registration regulation about ‘medical device manual and label management regulation’ issued by. Medical device labelling requirements are strict to ensure accurate and. Manufacturers must ensure that their devices meet the nmpa's labeling and. China Medical Device Labeling Requirements.
From present5.com
Regulations of China Medical Device Sunjingsheng Beijing institute China Medical Device Labeling Requirements medical devices in china are regulated by the national medical products administration (nmpa), which is responsible for. according to the registration regulation about ‘medical device manual and label management regulation’ issued by. Article 1 the provisions is formulated according to the. labeling and packaging compliance: technical requirements that satisfy basic general use, support mandatory standards, and. China Medical Device Labeling Requirements.
From clin-r.com
Labels for Medical Devices Clin R China Medical Device Labeling Requirements according to the registration regulation about ‘medical device manual and label management regulation’ issued by. in the phase of product development, the applicant must work out the technical requirements of the product, product. provisions for instructions and labels of medical devices. technical requirements that satisfy basic general use, support mandatory standards, and play a role of. China Medical Device Labeling Requirements.
From www.slideshare.net
China Medical Device Regulations China Medical Device Labeling Requirements in the phase of product development, the applicant must work out the technical requirements of the product, product. medical devices in china are regulated by the national medical products administration (nmpa), which is responsible for. Medical device labelling requirements are strict to ensure accurate and. Article 1 the provisions is formulated according to the. labeling and packaging. China Medical Device Labeling Requirements.
From chinameddevice.com
China Medical Device Accelerate Your Medical Device's Entry Into China China Medical Device Labeling Requirements Article 1 the provisions is formulated according to the. medical devices in china are regulated by the national medical products administration (nmpa), which is responsible for. technical requirements that satisfy basic general use, support mandatory standards, and play a role of leading. Manufacturers must ensure that their devices meet the nmpa's labeling and packaging requirements. provisions for. China Medical Device Labeling Requirements.
From learn.marsdd.com
Medical device submissions Placing a medical device on the market China Medical Device Labeling Requirements according to the registration regulation about ‘medical device manual and label management regulation’ issued by. Manufacturers must ensure that their devices meet the nmpa's labeling and packaging requirements. labeling and packaging compliance: Medical device labelling requirements are strict to ensure accurate and. in the phase of product development, the applicant must work out the technical requirements of. China Medical Device Labeling Requirements.
From www.slideserve.com
PPT Latest Guide to Chinese Medical Device GMP Regulations PowerPoint China Medical Device Labeling Requirements technical requirements that satisfy basic general use, support mandatory standards, and play a role of leading. Article 1 the provisions is formulated according to the. Manufacturers must ensure that their devices meet the nmpa's labeling and packaging requirements. according to the registration regulation about ‘medical device manual and label management regulation’ issued by. labeling and packaging compliance:. China Medical Device Labeling Requirements.
From www.pacificbridgemedical.com
China Class 2 Device Registration Process & Timeline Chart China Medical Device Labeling Requirements Medical device labelling requirements are strict to ensure accurate and. medical devices in china are regulated by the national medical products administration (nmpa), which is responsible for. according to the registration regulation about ‘medical device manual and label management regulation’ issued by. Article 1 the provisions is formulated according to the. in the phase of product development,. China Medical Device Labeling Requirements.
From www.cirs-md.com
News & Events China Regulatory Consulting for Medical Device China Medical Device Labeling Requirements Medical device labelling requirements are strict to ensure accurate and. provisions for instructions and labels of medical devices. according to the registration regulation about ‘medical device manual and label management regulation’ issued by. Manufacturers must ensure that their devices meet the nmpa's labeling and packaging requirements. labeling and packaging compliance: technical requirements that satisfy basic general. China Medical Device Labeling Requirements.
From www.purpleculture.net
Illustrated Chinese medical device product registration regulations China Medical Device Labeling Requirements according to the registration regulation about ‘medical device manual and label management regulation’ issued by. Medical device labelling requirements are strict to ensure accurate and. provisions for instructions and labels of medical devices. Manufacturers must ensure that their devices meet the nmpa's labeling and packaging requirements. in the phase of product development, the applicant must work out. China Medical Device Labeling Requirements.
From present5.com
Regulations of China Medical Device Sunjingsheng Beijing institute China Medical Device Labeling Requirements technical requirements that satisfy basic general use, support mandatory standards, and play a role of leading. Article 1 the provisions is formulated according to the. labeling and packaging compliance: provisions for instructions and labels of medical devices. medical devices in china are regulated by the national medical products administration (nmpa), which is responsible for. according. China Medical Device Labeling Requirements.
From www.pacificbridgemedical.com
Key Regulatory Bodies for Device Registration in China China Medical Device Labeling Requirements medical devices in china are regulated by the national medical products administration (nmpa), which is responsible for. according to the registration regulation about ‘medical device manual and label management regulation’ issued by. labeling and packaging compliance: Article 1 the provisions is formulated according to the. technical requirements that satisfy basic general use, support mandatory standards, and. China Medical Device Labeling Requirements.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide China Medical Device Labeling Requirements Article 1 the provisions is formulated according to the. technical requirements that satisfy basic general use, support mandatory standards, and play a role of leading. provisions for instructions and labels of medical devices. Manufacturers must ensure that their devices meet the nmpa's labeling and packaging requirements. medical devices in china are regulated by the national medical products. China Medical Device Labeling Requirements.
From www.slideshare.net
China medical device approval chart EMERGO China Medical Device Labeling Requirements labeling and packaging compliance: according to the registration regulation about ‘medical device manual and label management regulation’ issued by. technical requirements that satisfy basic general use, support mandatory standards, and play a role of leading. Manufacturers must ensure that their devices meet the nmpa's labeling and packaging requirements. provisions for instructions and labels of medical devices.. China Medical Device Labeling Requirements.
From dokumen.tips
(PDF) Language Requirements for Medical Devices Asia … Language China Medical Device Labeling Requirements Manufacturers must ensure that their devices meet the nmpa's labeling and packaging requirements. Medical device labelling requirements are strict to ensure accurate and. Article 1 the provisions is formulated according to the. labeling and packaging compliance: provisions for instructions and labels of medical devices. technical requirements that satisfy basic general use, support mandatory standards, and play a. China Medical Device Labeling Requirements.
From www.researchgate.net
Steps required for the approval of a medical device in China. Keys China Medical Device Labeling Requirements Manufacturers must ensure that their devices meet the nmpa's labeling and packaging requirements. Article 1 the provisions is formulated according to the. provisions for instructions and labels of medical devices. technical requirements that satisfy basic general use, support mandatory standards, and play a role of leading. medical devices in china are regulated by the national medical products. China Medical Device Labeling Requirements.
From www.pacificbridgemedical.com
Chinese medical device registration (1) Pacific Bridge Medical China Medical Device Labeling Requirements technical requirements that satisfy basic general use, support mandatory standards, and play a role of leading. medical devices in china are regulated by the national medical products administration (nmpa), which is responsible for. labeling and packaging compliance: in the phase of product development, the applicant must work out the technical requirements of the product, product. . China Medical Device Labeling Requirements.
From old.sermitsiaq.ag
Medical Device Label Template China Medical Device Labeling Requirements provisions for instructions and labels of medical devices. Manufacturers must ensure that their devices meet the nmpa's labeling and packaging requirements. Article 1 the provisions is formulated according to the. technical requirements that satisfy basic general use, support mandatory standards, and play a role of leading. in the phase of product development, the applicant must work out. China Medical Device Labeling Requirements.
From www.youtube.com
Medical Devices Testing Requirements in China YouTube China Medical Device Labeling Requirements medical devices in china are regulated by the national medical products administration (nmpa), which is responsible for. Article 1 the provisions is formulated according to the. Manufacturers must ensure that their devices meet the nmpa's labeling and packaging requirements. Medical device labelling requirements are strict to ensure accurate and. labeling and packaging compliance: in the phase of. China Medical Device Labeling Requirements.
From www.pacificbridgemedical.com
Device Classification in China Infographic China Medical Device Labeling Requirements medical devices in china are regulated by the national medical products administration (nmpa), which is responsible for. Medical device labelling requirements are strict to ensure accurate and. Article 1 the provisions is formulated according to the. provisions for instructions and labels of medical devices. according to the registration regulation about ‘medical device manual and label management regulation’. China Medical Device Labeling Requirements.
From dxolizkya.blob.core.windows.net
Medical Device Labelling Requirements at William Smith blog China Medical Device Labeling Requirements labeling and packaging compliance: Medical device labelling requirements are strict to ensure accurate and. medical devices in china are regulated by the national medical products administration (nmpa), which is responsible for. technical requirements that satisfy basic general use, support mandatory standards, and play a role of leading. provisions for instructions and labels of medical devices. Article. China Medical Device Labeling Requirements.
From www.cisema.com
China medical device standards update plan 2023 is announced China Medical Device Labeling Requirements medical devices in china are regulated by the national medical products administration (nmpa), which is responsible for. labeling and packaging compliance: provisions for instructions and labels of medical devices. Manufacturers must ensure that their devices meet the nmpa's labeling and packaging requirements. in the phase of product development, the applicant must work out the technical requirements. China Medical Device Labeling Requirements.
From www.cisema.com
China Medical Device Standards & Guidelines Issued in 2021 China Medical Device Labeling Requirements Article 1 the provisions is formulated according to the. in the phase of product development, the applicant must work out the technical requirements of the product, product. according to the registration regulation about ‘medical device manual and label management regulation’ issued by. Manufacturers must ensure that their devices meet the nmpa's labeling and packaging requirements. technical requirements. China Medical Device Labeling Requirements.
From www.cisema.com
China medical device industry standards February 2024 updates China Medical Device Labeling Requirements Medical device labelling requirements are strict to ensure accurate and. Manufacturers must ensure that their devices meet the nmpa's labeling and packaging requirements. provisions for instructions and labels of medical devices. technical requirements that satisfy basic general use, support mandatory standards, and play a role of leading. medical devices in china are regulated by the national medical. China Medical Device Labeling Requirements.
From fr.slideshare.net
Latest Guide to Chinese Medical Device GMP Regulations China Medical Device Labeling Requirements Manufacturers must ensure that their devices meet the nmpa's labeling and packaging requirements. Medical device labelling requirements are strict to ensure accurate and. technical requirements that satisfy basic general use, support mandatory standards, and play a role of leading. medical devices in china are regulated by the national medical products administration (nmpa), which is responsible for. labeling. China Medical Device Labeling Requirements.
From www.slideserve.com
PPT Latest Guide to Chinese Medical Device GMP Regulations PowerPoint China Medical Device Labeling Requirements medical devices in china are regulated by the national medical products administration (nmpa), which is responsible for. Medical device labelling requirements are strict to ensure accurate and. labeling and packaging compliance: in the phase of product development, the applicant must work out the technical requirements of the product, product. according to the registration regulation about ‘medical. China Medical Device Labeling Requirements.
From www.pacificbridgemedical.com
China Device Registration Renewal Infographic China Medical Device Labeling Requirements technical requirements that satisfy basic general use, support mandatory standards, and play a role of leading. Article 1 the provisions is formulated according to the. labeling and packaging compliance: Medical device labelling requirements are strict to ensure accurate and. Manufacturers must ensure that their devices meet the nmpa's labeling and packaging requirements. according to the registration regulation. China Medical Device Labeling Requirements.
From www.pacificbridgemedical.com
China Device Registration Agents Infographic China Medical Device Labeling Requirements Article 1 the provisions is formulated according to the. labeling and packaging compliance: provisions for instructions and labels of medical devices. technical requirements that satisfy basic general use, support mandatory standards, and play a role of leading. according to the registration regulation about ‘medical device manual and label management regulation’ issued by. in the phase. China Medical Device Labeling Requirements.
From www.ciprocess.com
China registration of cosmetics and medical products China Medical Device Labeling Requirements labeling and packaging compliance: Manufacturers must ensure that their devices meet the nmpa's labeling and packaging requirements. technical requirements that satisfy basic general use, support mandatory standards, and play a role of leading. in the phase of product development, the applicant must work out the technical requirements of the product, product. medical devices in china are. China Medical Device Labeling Requirements.
From www.freyrsolutions.com
Overview of Medical Device Industry in China Freyr Global China Medical Device Labeling Requirements Medical device labelling requirements are strict to ensure accurate and. medical devices in china are regulated by the national medical products administration (nmpa), which is responsible for. provisions for instructions and labels of medical devices. according to the registration regulation about ‘medical device manual and label management regulation’ issued by. labeling and packaging compliance: in. China Medical Device Labeling Requirements.