Chlorine Reaction Product . In the presence of aprotic solvent, the product is a vicinal dihalide, as shown here for the addition of chlorine to propene. chlorine is a dense gas with a density of 3.21 grams per liter. revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. this chapter treats the chemistry of oxidants used in disinfection and chemical oxidation during water. generate chlorine gas and investigate its reactions with water or halide ions in this class practical. In this experiment, students can use microscale apparatus to generate chlorine gas safely themselves in the open laboratory, investigating how it reacts with water and halide ions in solution. Chlorine changes from a gas into a liquid at. By comparison, the density of air is 1.29 grams per liter. addition reaction also occur easily between halogens (br 2 and cl 2) and alkenes. small amounts of chlorine can be produced in the lab by oxidizing \(hcl\) with \(mno_2\). The activity should take about 20 minutes. chlorine is produced at the titanium anode according to the following equation:
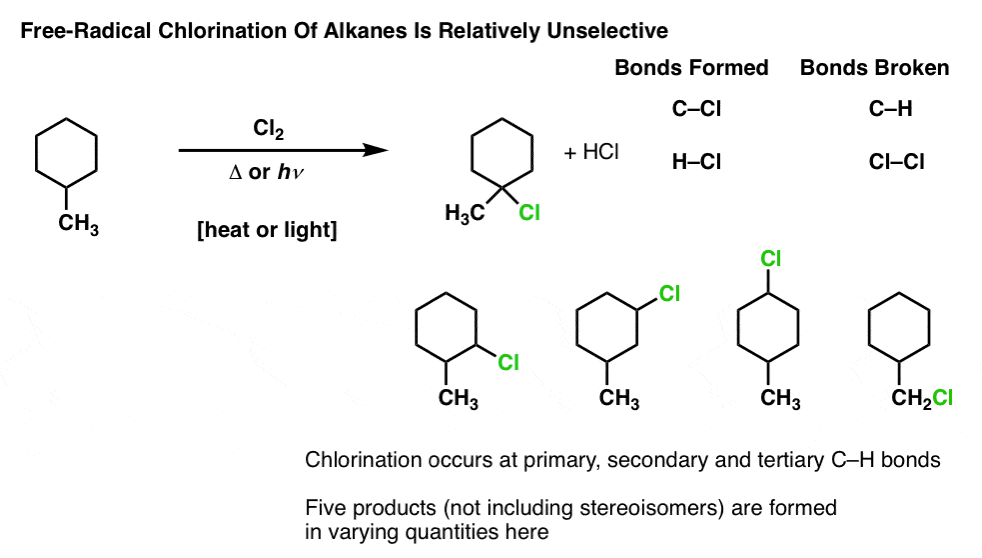
from www.masterorganicchemistry.com
addition reaction also occur easily between halogens (br 2 and cl 2) and alkenes. generate chlorine gas and investigate its reactions with water or halide ions in this class practical. revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. Chlorine changes from a gas into a liquid at. chlorine is a dense gas with a density of 3.21 grams per liter. In the presence of aprotic solvent, the product is a vicinal dihalide, as shown here for the addition of chlorine to propene. The activity should take about 20 minutes. this chapter treats the chemistry of oxidants used in disinfection and chemical oxidation during water. small amounts of chlorine can be produced in the lab by oxidizing \(hcl\) with \(mno_2\). In this experiment, students can use microscale apparatus to generate chlorine gas safely themselves in the open laboratory, investigating how it reacts with water and halide ions in solution.
Halogenation At Tiffany's Master Organic Chemistry
Chlorine Reaction Product addition reaction also occur easily between halogens (br 2 and cl 2) and alkenes. this chapter treats the chemistry of oxidants used in disinfection and chemical oxidation during water. revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. In the presence of aprotic solvent, the product is a vicinal dihalide, as shown here for the addition of chlorine to propene. chlorine is produced at the titanium anode according to the following equation: addition reaction also occur easily between halogens (br 2 and cl 2) and alkenes. chlorine is a dense gas with a density of 3.21 grams per liter. generate chlorine gas and investigate its reactions with water or halide ions in this class practical. small amounts of chlorine can be produced in the lab by oxidizing \(hcl\) with \(mno_2\). In this experiment, students can use microscale apparatus to generate chlorine gas safely themselves in the open laboratory, investigating how it reacts with water and halide ions in solution. Chlorine changes from a gas into a liquid at. The activity should take about 20 minutes. By comparison, the density of air is 1.29 grams per liter.
From www.masterorganicchemistry.com
Halogenation At Tiffany's Master Organic Chemistry Chlorine Reaction Product addition reaction also occur easily between halogens (br 2 and cl 2) and alkenes. chlorine is a dense gas with a density of 3.21 grams per liter. In the presence of aprotic solvent, the product is a vicinal dihalide, as shown here for the addition of chlorine to propene. By comparison, the density of air is 1.29 grams. Chlorine Reaction Product.
From www.youtube.com
Sodium and Chlorine ReactionMaking of Salt. YouTube Chlorine Reaction Product addition reaction also occur easily between halogens (br 2 and cl 2) and alkenes. In the presence of aprotic solvent, the product is a vicinal dihalide, as shown here for the addition of chlorine to propene. this chapter treats the chemistry of oxidants used in disinfection and chemical oxidation during water. generate chlorine gas and investigate its. Chlorine Reaction Product.
From www.researchgate.net
Chlorine evolution reaction performance (A) Schematic diagram of Chlorine Reaction Product chlorine is produced at the titanium anode according to the following equation: revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. In the presence of aprotic solvent, the product is a vicinal dihalide, as shown here for the addition of chlorine to propene. generate chlorine. Chlorine Reaction Product.
From www.toppr.com
Chlorobenzene is formed by reaction of chlorine with benzene in the Chlorine Reaction Product chlorine is produced at the titanium anode according to the following equation: addition reaction also occur easily between halogens (br 2 and cl 2) and alkenes. By comparison, the density of air is 1.29 grams per liter. this chapter treats the chemistry of oxidants used in disinfection and chemical oxidation during water. In this experiment, students can. Chlorine Reaction Product.
From dxoilsdmm.blob.core.windows.net
Urine And Chlorine Reaction at Lynn Quinones blog Chlorine Reaction Product The activity should take about 20 minutes. chlorine is produced at the titanium anode according to the following equation: In the presence of aprotic solvent, the product is a vicinal dihalide, as shown here for the addition of chlorine to propene. small amounts of chlorine can be produced in the lab by oxidizing \(hcl\) with \(mno_2\). Chlorine changes. Chlorine Reaction Product.
From exontsuic.blob.core.windows.net
Chlorine Gas Balanced Equation at Thomas Sheehan blog Chlorine Reaction Product revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. small amounts of chlorine can be produced in the lab by oxidizing \(hcl\) with \(mno_2\). Chlorine changes from a gas into a liquid at. this chapter treats the chemistry of oxidants used in disinfection and chemical. Chlorine Reaction Product.
From www.aquaprofessor.com
Why My Tap Water Smells Like Chlorine? (How To Fix It) Chlorine Reaction Product generate chlorine gas and investigate its reactions with water or halide ions in this class practical. In the presence of aprotic solvent, the product is a vicinal dihalide, as shown here for the addition of chlorine to propene. addition reaction also occur easily between halogens (br 2 and cl 2) and alkenes. chlorine is a dense gas. Chlorine Reaction Product.
From openoregon.pressbooks.pub
4.3 AcidBase Reactions Introduction to Chemistry Chlorine Reaction Product Chlorine changes from a gas into a liquid at. chlorine is a dense gas with a density of 3.21 grams per liter. generate chlorine gas and investigate its reactions with water or halide ions in this class practical. The activity should take about 20 minutes. addition reaction also occur easily between halogens (br 2 and cl 2). Chlorine Reaction Product.
From www.nagwa.com
Question Video Naming the Product of the Addition Reaction of Ethene Chlorine Reaction Product By comparison, the density of air is 1.29 grams per liter. In the presence of aprotic solvent, the product is a vicinal dihalide, as shown here for the addition of chlorine to propene. generate chlorine gas and investigate its reactions with water or halide ions in this class practical. chlorine is produced at the titanium anode according to. Chlorine Reaction Product.
From www.science-revision.co.uk
Free radical subsititution Chlorine Reaction Product The activity should take about 20 minutes. In the presence of aprotic solvent, the product is a vicinal dihalide, as shown here for the addition of chlorine to propene. small amounts of chlorine can be produced in the lab by oxidizing \(hcl\) with \(mno_2\). addition reaction also occur easily between halogens (br 2 and cl 2) and alkenes.. Chlorine Reaction Product.
From www.youtube.com
Chlorine and Water AS Chemistry YouTube Chlorine Reaction Product revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. this chapter treats the chemistry of oxidants used in disinfection and chemical oxidation during water. chlorine is a dense gas with a density of 3.21 grams per liter. The activity should take about 20 minutes. In. Chlorine Reaction Product.
From www.masterorganicchemistry.com
Synthesis (2) Reactions of Alkanes Master Organic Chemistry Chlorine Reaction Product The activity should take about 20 minutes. generate chlorine gas and investigate its reactions with water or halide ions in this class practical. By comparison, the density of air is 1.29 grams per liter. chlorine is a dense gas with a density of 3.21 grams per liter. chlorine is produced at the titanium anode according to the. Chlorine Reaction Product.
From www.numerade.com
⏩SOLVEDThe reaction of chlorine with pentane gives a mixture of Chlorine Reaction Product this chapter treats the chemistry of oxidants used in disinfection and chemical oxidation during water. revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. generate chlorine gas and investigate its reactions with water or halide ions in this class practical. In the presence of aprotic. Chlorine Reaction Product.
From www.youtube.com
Sodium and Chlorine Reaction YouTube Chlorine Reaction Product revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. By comparison, the density of air is 1.29 grams per liter. chlorine is produced at the titanium anode according to the following equation: In the presence of aprotic solvent, the product is a vicinal dihalide, as shown. Chlorine Reaction Product.
From spmchemistry.blog.onlinetuition.com.my
Reaction of Alkali Metals with Chlorine SPM Chemistry Chlorine Reaction Product chlorine is a dense gas with a density of 3.21 grams per liter. By comparison, the density of air is 1.29 grams per liter. chlorine is produced at the titanium anode according to the following equation: small amounts of chlorine can be produced in the lab by oxidizing \(hcl\) with \(mno_2\). Chlorine changes from a gas into. Chlorine Reaction Product.
From www.youtube.com
How to Balance NaOH + Cl2 = NaCl + NaClO + H2O (Dilute Sodium hydroxide Chlorine Reaction Product generate chlorine gas and investigate its reactions with water or halide ions in this class practical. this chapter treats the chemistry of oxidants used in disinfection and chemical oxidation during water. small amounts of chlorine can be produced in the lab by oxidizing \(hcl\) with \(mno_2\). revision notes on the reactions of chlorine for the cie. Chlorine Reaction Product.
From ar.inspiredpencil.com
Chlorine Diagram Chlorine Reaction Product In this experiment, students can use microscale apparatus to generate chlorine gas safely themselves in the open laboratory, investigating how it reacts with water and halide ions in solution. revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. small amounts of chlorine can be produced in. Chlorine Reaction Product.
From www.chegg.com
Solved Chlorine and bromine react in the dark with alkenes. Chlorine Reaction Product this chapter treats the chemistry of oxidants used in disinfection and chemical oxidation during water. The activity should take about 20 minutes. In this experiment, students can use microscale apparatus to generate chlorine gas safely themselves in the open laboratory, investigating how it reacts with water and halide ions in solution. In the presence of aprotic solvent, the product. Chlorine Reaction Product.
From www.researchgate.net
Simplified chemical reaction scheme of the generation of chlorine Chlorine Reaction Product addition reaction also occur easily between halogens (br 2 and cl 2) and alkenes. chlorine is produced at the titanium anode according to the following equation: In this experiment, students can use microscale apparatus to generate chlorine gas safely themselves in the open laboratory, investigating how it reacts with water and halide ions in solution. By comparison, the. Chlorine Reaction Product.
From www.britannica.com
Chemical reaction The BrønstedLowry theory Britannica Chlorine Reaction Product Chlorine changes from a gas into a liquid at. In this experiment, students can use microscale apparatus to generate chlorine gas safely themselves in the open laboratory, investigating how it reacts with water and halide ions in solution. this chapter treats the chemistry of oxidants used in disinfection and chemical oxidation during water. By comparison, the density of air. Chlorine Reaction Product.
From www.youtube.com
Synthesizing and Condensing Chlorine + Liquid Chlorine and Sodium Chlorine Reaction Product Chlorine changes from a gas into a liquid at. By comparison, the density of air is 1.29 grams per liter. revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. In the presence of aprotic solvent, the product is a vicinal dihalide, as shown here for the addition. Chlorine Reaction Product.
From www.dreamstime.com
Chlorine in Bottle, Chemical in the Laboratory Stock Image Image of Chlorine Reaction Product this chapter treats the chemistry of oxidants used in disinfection and chemical oxidation during water. small amounts of chlorine can be produced in the lab by oxidizing \(hcl\) with \(mno_2\). By comparison, the density of air is 1.29 grams per liter. The activity should take about 20 minutes. revision notes on the reactions of chlorine for the. Chlorine Reaction Product.
From www.lovibond.com
Reagent Set Total Chlorine Lovibond Chlorine Reaction Product In this experiment, students can use microscale apparatus to generate chlorine gas safely themselves in the open laboratory, investigating how it reacts with water and halide ions in solution. Chlorine changes from a gas into a liquid at. generate chlorine gas and investigate its reactions with water or halide ions in this class practical. chlorine is produced at. Chlorine Reaction Product.
From www.essentialchemicalindustry.org
Chlorine Chlorine Reaction Product In the presence of aprotic solvent, the product is a vicinal dihalide, as shown here for the addition of chlorine to propene. chlorine is a dense gas with a density of 3.21 grams per liter. generate chlorine gas and investigate its reactions with water or halide ions in this class practical. Chlorine changes from a gas into a. Chlorine Reaction Product.
From www.numerade.com
SOLVED Chlorine and bromine react In the dark WIth alkenes The Chlorine Reaction Product chlorine is produced at the titanium anode according to the following equation: In the presence of aprotic solvent, the product is a vicinal dihalide, as shown here for the addition of chlorine to propene. Chlorine changes from a gas into a liquid at. small amounts of chlorine can be produced in the lab by oxidizing \(hcl\) with \(mno_2\).. Chlorine Reaction Product.
From www.slideserve.com
PPT Chlorination of Propane PowerPoint Presentation, free download Chlorine Reaction Product small amounts of chlorine can be produced in the lab by oxidizing \(hcl\) with \(mno_2\). addition reaction also occur easily between halogens (br 2 and cl 2) and alkenes. generate chlorine gas and investigate its reactions with water or halide ions in this class practical. In this experiment, students can use microscale apparatus to generate chlorine gas. Chlorine Reaction Product.
From www.slideserve.com
PPT Chapter 4—An Introduction to Organic Reactions PowerPoint Chlorine Reaction Product revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. chlorine is a dense gas with a density of 3.21 grams per liter. Chlorine changes from a gas into a liquid at. By comparison, the density of air is 1.29 grams per liter. small amounts of. Chlorine Reaction Product.
From www.youtube.com
Balancing and Writing the Equation for Sodium + Chlorine gas YouTube Chlorine Reaction Product chlorine is a dense gas with a density of 3.21 grams per liter. In this experiment, students can use microscale apparatus to generate chlorine gas safely themselves in the open laboratory, investigating how it reacts with water and halide ions in solution. In the presence of aprotic solvent, the product is a vicinal dihalide, as shown here for the. Chlorine Reaction Product.
From www.slideshare.net
Energy changes Chlorine Reaction Product chlorine is a dense gas with a density of 3.21 grams per liter. In this experiment, students can use microscale apparatus to generate chlorine gas safely themselves in the open laboratory, investigating how it reacts with water and halide ions in solution. revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by. Chlorine Reaction Product.
From www.onlinemathlearning.com
Chemical Reactions IGCSE Chemistry (solutions, examples, worksheets Chlorine Reaction Product In this experiment, students can use microscale apparatus to generate chlorine gas safely themselves in the open laboratory, investigating how it reacts with water and halide ions in solution. In the presence of aprotic solvent, the product is a vicinal dihalide, as shown here for the addition of chlorine to propene. Chlorine changes from a gas into a liquid at.. Chlorine Reaction Product.
From www.chemistrystudent.com
Alkanes Free Radical Substitution (ALevel) ChemistryStudent Chlorine Reaction Product The activity should take about 20 minutes. small amounts of chlorine can be produced in the lab by oxidizing \(hcl\) with \(mno_2\). In this experiment, students can use microscale apparatus to generate chlorine gas safely themselves in the open laboratory, investigating how it reacts with water and halide ions in solution. revision notes on the reactions of chlorine. Chlorine Reaction Product.
From www.youtube.com
Type of Reaction for Na + Cl2 = NaCl (Sodium + Chlorine gas) YouTube Chlorine Reaction Product Chlorine changes from a gas into a liquid at. this chapter treats the chemistry of oxidants used in disinfection and chemical oxidation during water. addition reaction also occur easily between halogens (br 2 and cl 2) and alkenes. chlorine is produced at the titanium anode according to the following equation: small amounts of chlorine can be. Chlorine Reaction Product.
From www.sciencephoto.com
Ironchlorine reaction Stock Image C011/4535 Science Photo Library Chlorine Reaction Product revision notes on the reactions of chlorine for the cie a level chemistry syllabus, written by the chemistry experts at save. Chlorine changes from a gas into a liquid at. chlorine is produced at the titanium anode according to the following equation: small amounts of chlorine can be produced in the lab by oxidizing \(hcl\) with \(mno_2\).. Chlorine Reaction Product.
From mavink.com
Ethene And Chlorine Reaction Chlorine Reaction Product small amounts of chlorine can be produced in the lab by oxidizing \(hcl\) with \(mno_2\). chlorine is a dense gas with a density of 3.21 grams per liter. addition reaction also occur easily between halogens (br 2 and cl 2) and alkenes. generate chlorine gas and investigate its reactions with water or halide ions in this. Chlorine Reaction Product.
From www.fuelcellstore.com
Chloralkali Production Membranes Chlorine Reaction Product chlorine is a dense gas with a density of 3.21 grams per liter. By comparison, the density of air is 1.29 grams per liter. small amounts of chlorine can be produced in the lab by oxidizing \(hcl\) with \(mno_2\). this chapter treats the chemistry of oxidants used in disinfection and chemical oxidation during water. chlorine is. Chlorine Reaction Product.