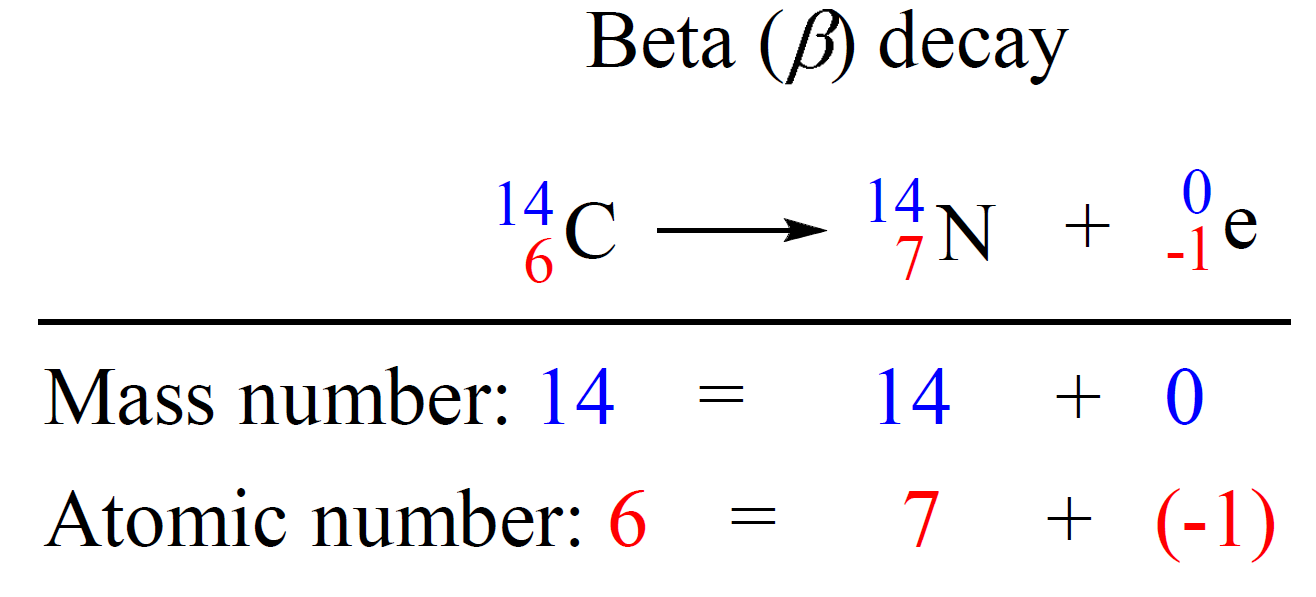
Alpha Beta Gamma Decay Formula . The most common are protons, neutrons, alpha particles, beta particles, positrons, and gamma rays, as shown in table \(\pageindex{1}\). The three main types of radioactive particle or radiation are: “type,” “nuclear equation,” “representation,” and “change in mass / atomic numbers.” under the “type”. The first row is a header row and it labels each column: To become more stable, a nucleus can emit particles or radiation by the process of radioactive decay; 238 = 4 + 234 238 = 4. Formula and calculation of radioactive decay the. Gamma decay often accompanies other types of decay, such as alpha and beta, to release excess energy from the nucleus. In this chapter we consider the other two. Principles of radioactive decay in section 1.3 and we studied more in depth alpha decay in section 3.3. With the wrong number of neutrons, nuclei can fall apart. In the alpha decay of u238 u 238 (equation 17.3.1 17.3.1), both atomic and mass numbers are conserved:
from general.chemistrysteps.com
In the alpha decay of u238 u 238 (equation 17.3.1 17.3.1), both atomic and mass numbers are conserved: In this chapter we consider the other two. Principles of radioactive decay in section 1.3 and we studied more in depth alpha decay in section 3.3. “type,” “nuclear equation,” “representation,” and “change in mass / atomic numbers.” under the “type”. To become more stable, a nucleus can emit particles or radiation by the process of radioactive decay; Formula and calculation of radioactive decay the. With the wrong number of neutrons, nuclei can fall apart. Gamma decay often accompanies other types of decay, such as alpha and beta, to release excess energy from the nucleus. The most common are protons, neutrons, alpha particles, beta particles, positrons, and gamma rays, as shown in table \(\pageindex{1}\). The first row is a header row and it labels each column:
Balancing Nuclear Reactions Chemistry Steps
Alpha Beta Gamma Decay Formula The three main types of radioactive particle or radiation are: Principles of radioactive decay in section 1.3 and we studied more in depth alpha decay in section 3.3. To become more stable, a nucleus can emit particles or radiation by the process of radioactive decay; The most common are protons, neutrons, alpha particles, beta particles, positrons, and gamma rays, as shown in table \(\pageindex{1}\). “type,” “nuclear equation,” “representation,” and “change in mass / atomic numbers.” under the “type”. In the alpha decay of u238 u 238 (equation 17.3.1 17.3.1), both atomic and mass numbers are conserved: In this chapter we consider the other two. Formula and calculation of radioactive decay the. 238 = 4 + 234 238 = 4. The first row is a header row and it labels each column: The three main types of radioactive particle or radiation are: With the wrong number of neutrons, nuclei can fall apart. Gamma decay often accompanies other types of decay, such as alpha and beta, to release excess energy from the nucleus.
From general.chemistrysteps.com
Balancing Nuclear Reactions Chemistry Steps Alpha Beta Gamma Decay Formula The three main types of radioactive particle or radiation are: “type,” “nuclear equation,” “representation,” and “change in mass / atomic numbers.” under the “type”. Principles of radioactive decay in section 1.3 and we studied more in depth alpha decay in section 3.3. 238 = 4 + 234 238 = 4. The first row is a header row and it labels. Alpha Beta Gamma Decay Formula.
From learningdbhimmel.z19.web.core.windows.net
Nuclear Chemistry Alpha Beta Gamma Decay Practice Worksheet Alpha Beta Gamma Decay Formula 238 = 4 + 234 238 = 4. The most common are protons, neutrons, alpha particles, beta particles, positrons, and gamma rays, as shown in table \(\pageindex{1}\). The first row is a header row and it labels each column: With the wrong number of neutrons, nuclei can fall apart. Principles of radioactive decay in section 1.3 and we studied more. Alpha Beta Gamma Decay Formula.
From goc-oivf2.blogspot.com
40 alpha beta gamma decay worksheet Worksheet Information Alpha Beta Gamma Decay Formula The three main types of radioactive particle or radiation are: Formula and calculation of radioactive decay the. To become more stable, a nucleus can emit particles or radiation by the process of radioactive decay; Principles of radioactive decay in section 1.3 and we studied more in depth alpha decay in section 3.3. “type,” “nuclear equation,” “representation,” and “change in mass. Alpha Beta Gamma Decay Formula.
From modernphysicsls.blogspot.com
Beta decay Alpha Beta Gamma Decay Formula Gamma decay often accompanies other types of decay, such as alpha and beta, to release excess energy from the nucleus. In this chapter we consider the other two. The three main types of radioactive particle or radiation are: To become more stable, a nucleus can emit particles or radiation by the process of radioactive decay; Formula and calculation of radioactive. Alpha Beta Gamma Decay Formula.
From animalia-life.club
Alpha Beta Gamma Decay Alpha Beta Gamma Decay Formula 238 = 4 + 234 238 = 4. Formula and calculation of radioactive decay the. The most common are protons, neutrons, alpha particles, beta particles, positrons, and gamma rays, as shown in table \(\pageindex{1}\). With the wrong number of neutrons, nuclei can fall apart. To become more stable, a nucleus can emit particles or radiation by the process of radioactive. Alpha Beta Gamma Decay Formula.
From animalia-life.club
Alpha Beta Gamma Decay Alpha Beta Gamma Decay Formula Formula and calculation of radioactive decay the. Principles of radioactive decay in section 1.3 and we studied more in depth alpha decay in section 3.3. 238 = 4 + 234 238 = 4. The first row is a header row and it labels each column: With the wrong number of neutrons, nuclei can fall apart. In the alpha decay of. Alpha Beta Gamma Decay Formula.
From animalia-life.club
Alpha Beta Gamma Decay Alpha Beta Gamma Decay Formula Gamma decay often accompanies other types of decay, such as alpha and beta, to release excess energy from the nucleus. To become more stable, a nucleus can emit particles or radiation by the process of radioactive decay; With the wrong number of neutrons, nuclei can fall apart. The three main types of radioactive particle or radiation are: The most common. Alpha Beta Gamma Decay Formula.
From radioactivedecay9.weebly.com
What is Radioactive Decay? Radioactive Decay Alpha Beta Gamma Decay Formula Gamma decay often accompanies other types of decay, such as alpha and beta, to release excess energy from the nucleus. “type,” “nuclear equation,” “representation,” and “change in mass / atomic numbers.” under the “type”. With the wrong number of neutrons, nuclei can fall apart. In this chapter we consider the other two. The most common are protons, neutrons, alpha particles,. Alpha Beta Gamma Decay Formula.
From www.vrogue.co
Alpha Beta Decay Equations Revision Worksheet Docx Al vrogue.co Alpha Beta Gamma Decay Formula In the alpha decay of u238 u 238 (equation 17.3.1 17.3.1), both atomic and mass numbers are conserved: To become more stable, a nucleus can emit particles or radiation by the process of radioactive decay; Formula and calculation of radioactive decay the. With the wrong number of neutrons, nuclei can fall apart. In this chapter we consider the other two.. Alpha Beta Gamma Decay Formula.
From aboutradiation.blogspot.com
Alpha Beta And Gamma Radiation Pdf All About Radiation Alpha Beta Gamma Decay Formula In this chapter we consider the other two. The first row is a header row and it labels each column: The three main types of radioactive particle or radiation are: Gamma decay often accompanies other types of decay, such as alpha and beta, to release excess energy from the nucleus. Principles of radioactive decay in section 1.3 and we studied. Alpha Beta Gamma Decay Formula.
From mmerevise.co.uk
Alpha, Beta and Gamma Radiation Worksheets, Questions and Revision MME Alpha Beta Gamma Decay Formula Principles of radioactive decay in section 1.3 and we studied more in depth alpha decay in section 3.3. The most common are protons, neutrons, alpha particles, beta particles, positrons, and gamma rays, as shown in table \(\pageindex{1}\). In the alpha decay of u238 u 238 (equation 17.3.1 17.3.1), both atomic and mass numbers are conserved: Gamma decay often accompanies other. Alpha Beta Gamma Decay Formula.
From general.chemistrysteps.com
Balancing Nuclear Reactions Chemistry Steps Alpha Beta Gamma Decay Formula To become more stable, a nucleus can emit particles or radiation by the process of radioactive decay; In the alpha decay of u238 u 238 (equation 17.3.1 17.3.1), both atomic and mass numbers are conserved: With the wrong number of neutrons, nuclei can fall apart. Gamma decay often accompanies other types of decay, such as alpha and beta, to release. Alpha Beta Gamma Decay Formula.
From animalia-life.club
Alpha Beta Gamma Decay Alpha Beta Gamma Decay Formula Principles of radioactive decay in section 1.3 and we studied more in depth alpha decay in section 3.3. 238 = 4 + 234 238 = 4. Formula and calculation of radioactive decay the. “type,” “nuclear equation,” “representation,” and “change in mass / atomic numbers.” under the “type”. Gamma decay often accompanies other types of decay, such as alpha and beta,. Alpha Beta Gamma Decay Formula.
From ar.inspiredpencil.com
Gamma Decay Equation Alpha Beta Gamma Decay Formula Gamma decay often accompanies other types of decay, such as alpha and beta, to release excess energy from the nucleus. In the alpha decay of u238 u 238 (equation 17.3.1 17.3.1), both atomic and mass numbers are conserved: Principles of radioactive decay in section 1.3 and we studied more in depth alpha decay in section 3.3. In this chapter we. Alpha Beta Gamma Decay Formula.
From www.slideshare.net
Alpha beta and gamma decay equations Alpha Beta Gamma Decay Formula In this chapter we consider the other two. Gamma decay often accompanies other types of decay, such as alpha and beta, to release excess energy from the nucleus. “type,” “nuclear equation,” “representation,” and “change in mass / atomic numbers.” under the “type”. With the wrong number of neutrons, nuclei can fall apart. To become more stable, a nucleus can emit. Alpha Beta Gamma Decay Formula.
From animalia-life.club
Alpha Beta Gamma Decay Alpha Beta Gamma Decay Formula The three main types of radioactive particle or radiation are: In the alpha decay of u238 u 238 (equation 17.3.1 17.3.1), both atomic and mass numbers are conserved: To become more stable, a nucleus can emit particles or radiation by the process of radioactive decay; The most common are protons, neutrons, alpha particles, beta particles, positrons, and gamma rays, as. Alpha Beta Gamma Decay Formula.
From ar.inspiredpencil.com
Alpha Beta Gamma Decay Alpha Beta Gamma Decay Formula “type,” “nuclear equation,” “representation,” and “change in mass / atomic numbers.” under the “type”. In this chapter we consider the other two. To become more stable, a nucleus can emit particles or radiation by the process of radioactive decay; Formula and calculation of radioactive decay the. In the alpha decay of u238 u 238 (equation 17.3.1 17.3.1), both atomic and. Alpha Beta Gamma Decay Formula.
From www.youtube.com
Different types of decay Alpha vs. Beta vs. Gamma decay Visual Alpha Beta Gamma Decay Formula Gamma decay often accompanies other types of decay, such as alpha and beta, to release excess energy from the nucleus. Principles of radioactive decay in section 1.3 and we studied more in depth alpha decay in section 3.3. Formula and calculation of radioactive decay the. In the alpha decay of u238 u 238 (equation 17.3.1 17.3.1), both atomic and mass. Alpha Beta Gamma Decay Formula.
From www.youtube.com
Alpha, Beta and Gamma Decay Types of Radioactive Decay Alpha Beta Gamma Decay Formula The three main types of radioactive particle or radiation are: Gamma decay often accompanies other types of decay, such as alpha and beta, to release excess energy from the nucleus. With the wrong number of neutrons, nuclei can fall apart. 238 = 4 + 234 238 = 4. The most common are protons, neutrons, alpha particles, beta particles, positrons, and. Alpha Beta Gamma Decay Formula.
From ar.inspiredpencil.com
Alpha Beta Gamma Decay Alpha Beta Gamma Decay Formula Principles of radioactive decay in section 1.3 and we studied more in depth alpha decay in section 3.3. The most common are protons, neutrons, alpha particles, beta particles, positrons, and gamma rays, as shown in table \(\pageindex{1}\). With the wrong number of neutrons, nuclei can fall apart. The first row is a header row and it labels each column: To. Alpha Beta Gamma Decay Formula.
From sciencedrill.com
A Guide to Alpha Decay Including Equations and Uses Science Drill Alpha Beta Gamma Decay Formula 238 = 4 + 234 238 = 4. The first row is a header row and it labels each column: Formula and calculation of radioactive decay the. “type,” “nuclear equation,” “representation,” and “change in mass / atomic numbers.” under the “type”. To become more stable, a nucleus can emit particles or radiation by the process of radioactive decay; In this. Alpha Beta Gamma Decay Formula.
From animalia-life.club
Alpha Beta Gamma Decay Alpha Beta Gamma Decay Formula In this chapter we consider the other two. To become more stable, a nucleus can emit particles or radiation by the process of radioactive decay; With the wrong number of neutrons, nuclei can fall apart. The most common are protons, neutrons, alpha particles, beta particles, positrons, and gamma rays, as shown in table \(\pageindex{1}\). In the alpha decay of u238. Alpha Beta Gamma Decay Formula.
From www.equationsworksheets.net
Radioactive Decay Equations Worksheet Equations Worksheets Alpha Beta Gamma Decay Formula In the alpha decay of u238 u 238 (equation 17.3.1 17.3.1), both atomic and mass numbers are conserved: The most common are protons, neutrons, alpha particles, beta particles, positrons, and gamma rays, as shown in table \(\pageindex{1}\). To become more stable, a nucleus can emit particles or radiation by the process of radioactive decay; Gamma decay often accompanies other types. Alpha Beta Gamma Decay Formula.
From www.wou.edu
CH103 CHAPTER 3 Radioactivity and Nuclear Chemistry Chemistry Alpha Beta Gamma Decay Formula Formula and calculation of radioactive decay the. Gamma decay often accompanies other types of decay, such as alpha and beta, to release excess energy from the nucleus. In the alpha decay of u238 u 238 (equation 17.3.1 17.3.1), both atomic and mass numbers are conserved: In this chapter we consider the other two. “type,” “nuclear equation,” “representation,” and “change in. Alpha Beta Gamma Decay Formula.
From www.sliderbase.com
Beta Decay Alpha Beta Gamma Decay Formula In this chapter we consider the other two. To become more stable, a nucleus can emit particles or radiation by the process of radioactive decay; In the alpha decay of u238 u 238 (equation 17.3.1 17.3.1), both atomic and mass numbers are conserved: Gamma decay often accompanies other types of decay, such as alpha and beta, to release excess energy. Alpha Beta Gamma Decay Formula.
From stock.adobe.com
Alpha decay, beta decay and gamma decay equations. Nuclear chemistry Alpha Beta Gamma Decay Formula The most common are protons, neutrons, alpha particles, beta particles, positrons, and gamma rays, as shown in table \(\pageindex{1}\). In the alpha decay of u238 u 238 (equation 17.3.1 17.3.1), both atomic and mass numbers are conserved: Formula and calculation of radioactive decay the. In this chapter we consider the other two. The three main types of radioactive particle or. Alpha Beta Gamma Decay Formula.
From games.udlvirtual.edu.pe
What Is Gamma Decay BEST GAMES WALKTHROUGH Alpha Beta Gamma Decay Formula “type,” “nuclear equation,” “representation,” and “change in mass / atomic numbers.” under the “type”. The most common are protons, neutrons, alpha particles, beta particles, positrons, and gamma rays, as shown in table \(\pageindex{1}\). The first row is a header row and it labels each column: In the alpha decay of u238 u 238 (equation 17.3.1 17.3.1), both atomic and mass. Alpha Beta Gamma Decay Formula.
From www.myxxgirl.com
Radioactive Decay Equation Alpha Beta Gamma Lesson Radioactive My XXX Alpha Beta Gamma Decay Formula Formula and calculation of radioactive decay the. 238 = 4 + 234 238 = 4. Principles of radioactive decay in section 1.3 and we studied more in depth alpha decay in section 3.3. In this chapter we consider the other two. “type,” “nuclear equation,” “representation,” and “change in mass / atomic numbers.” under the “type”. In the alpha decay of. Alpha Beta Gamma Decay Formula.
From animalia-life.club
Alpha Beta Gamma Decay Alpha Beta Gamma Decay Formula “type,” “nuclear equation,” “representation,” and “change in mass / atomic numbers.” under the “type”. In the alpha decay of u238 u 238 (equation 17.3.1 17.3.1), both atomic and mass numbers are conserved: 238 = 4 + 234 238 = 4. Principles of radioactive decay in section 1.3 and we studied more in depth alpha decay in section 3.3. To become. Alpha Beta Gamma Decay Formula.
From ar.inspiredpencil.com
Gamma Decay Equation Alpha Beta Gamma Decay Formula Gamma decay often accompanies other types of decay, such as alpha and beta, to release excess energy from the nucleus. Formula and calculation of radioactive decay the. Principles of radioactive decay in section 1.3 and we studied more in depth alpha decay in section 3.3. With the wrong number of neutrons, nuclei can fall apart. In the alpha decay of. Alpha Beta Gamma Decay Formula.
From animalia-life.club
Alpha Beta Gamma Decay Alpha Beta Gamma Decay Formula 238 = 4 + 234 238 = 4. The most common are protons, neutrons, alpha particles, beta particles, positrons, and gamma rays, as shown in table \(\pageindex{1}\). To become more stable, a nucleus can emit particles or radiation by the process of radioactive decay; Formula and calculation of radioactive decay the. With the wrong number of neutrons, nuclei can fall. Alpha Beta Gamma Decay Formula.