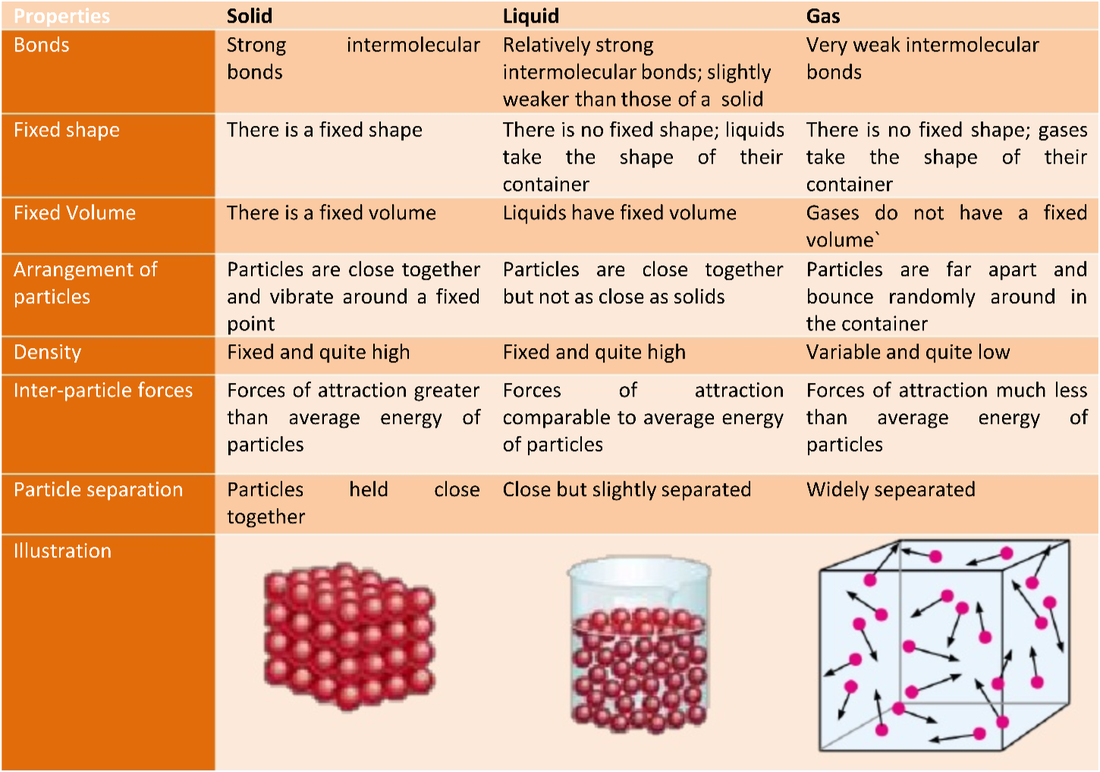
Kinetic Theory Of Solid Liquid And Gas . The kinetic molecular theory of matter is a concept that basically states that matter is composed of a very large number of very tiny. The theory assumes that gases consist of widely. Gases do not have a fixed volume, and, like liquids, take up the shape of the container. This page takes a simple look at solids, liquids and gases, and changes of state such as melting and boiling, in terms of the behaviour of the particles. Study how it interacts with gas laws, and view examples. Understand kinetic molecular theory and see how it explains the kinetic energy in solids, liquids, & gases. Describe the behavior of an ideal gas. Gases have a very low density. The kinetic molecular theory is a simple but very effective model that effectively explains ideal gas behavior. An introduction to kinetic theory. We gain a better understanding of pressure and temperature from the kinetic theory of gases, which assumes that atoms and molecules are in continuous random motion.
from igcsechemistryrevision.weebly.com
Understand kinetic molecular theory and see how it explains the kinetic energy in solids, liquids, & gases. The kinetic molecular theory of matter is a concept that basically states that matter is composed of a very large number of very tiny. The kinetic molecular theory is a simple but very effective model that effectively explains ideal gas behavior. Study how it interacts with gas laws, and view examples. Gases have a very low density. This page takes a simple look at solids, liquids and gases, and changes of state such as melting and boiling, in terms of the behaviour of the particles. An introduction to kinetic theory. Gases do not have a fixed volume, and, like liquids, take up the shape of the container. Describe the behavior of an ideal gas. We gain a better understanding of pressure and temperature from the kinetic theory of gases, which assumes that atoms and molecules are in continuous random motion.
1.1 Understand the arrangement, movement and energy of particles in
Kinetic Theory Of Solid Liquid And Gas The kinetic molecular theory is a simple but very effective model that effectively explains ideal gas behavior. Gases have a very low density. We gain a better understanding of pressure and temperature from the kinetic theory of gases, which assumes that atoms and molecules are in continuous random motion. Describe the behavior of an ideal gas. Study how it interacts with gas laws, and view examples. Gases do not have a fixed volume, and, like liquids, take up the shape of the container. The theory assumes that gases consist of widely. The kinetic molecular theory of matter is a concept that basically states that matter is composed of a very large number of very tiny. The kinetic molecular theory is a simple but very effective model that effectively explains ideal gas behavior. An introduction to kinetic theory. This page takes a simple look at solids, liquids and gases, and changes of state such as melting and boiling, in terms of the behaviour of the particles. Understand kinetic molecular theory and see how it explains the kinetic energy in solids, liquids, & gases.
From byjus.com
Explain briefly interconversion of states of matter on the basis of Kinetic Theory Of Solid Liquid And Gas The theory assumes that gases consist of widely. Study how it interacts with gas laws, and view examples. Gases have a very low density. Understand kinetic molecular theory and see how it explains the kinetic energy in solids, liquids, & gases. Describe the behavior of an ideal gas. We gain a better understanding of pressure and temperature from the kinetic. Kinetic Theory Of Solid Liquid And Gas.
From www.youtube.com
Model of molecules arrangement/solid liquid gas/states of matter/kansal Kinetic Theory Of Solid Liquid And Gas Study how it interacts with gas laws, and view examples. Gases have a very low density. Describe the behavior of an ideal gas. An introduction to kinetic theory. We gain a better understanding of pressure and temperature from the kinetic theory of gases, which assumes that atoms and molecules are in continuous random motion. This page takes a simple look. Kinetic Theory Of Solid Liquid And Gas.
From www.youtube.com
States of Matter Solid Liquid Gas States of Matter drawing Different Kinetic Theory Of Solid Liquid And Gas Study how it interacts with gas laws, and view examples. The kinetic molecular theory is a simple but very effective model that effectively explains ideal gas behavior. We gain a better understanding of pressure and temperature from the kinetic theory of gases, which assumes that atoms and molecules are in continuous random motion. Describe the behavior of an ideal gas.. Kinetic Theory Of Solid Liquid And Gas.
From www.elevise.co.uk
P3 A) The Particle Model AQA Combined Science Trilogy Elevise Kinetic Theory Of Solid Liquid And Gas The theory assumes that gases consist of widely. Gases do not have a fixed volume, and, like liquids, take up the shape of the container. The kinetic molecular theory is a simple but very effective model that effectively explains ideal gas behavior. Understand kinetic molecular theory and see how it explains the kinetic energy in solids, liquids, & gases. An. Kinetic Theory Of Solid Liquid And Gas.
From www.youtube.com
theory of Solids, Liquids & Gases IGCSE Chemistry Learn Kinetic Theory Of Solid Liquid And Gas An introduction to kinetic theory. This page takes a simple look at solids, liquids and gases, and changes of state such as melting and boiling, in terms of the behaviour of the particles. Understand kinetic molecular theory and see how it explains the kinetic energy in solids, liquids, & gases. We gain a better understanding of pressure and temperature from. Kinetic Theory Of Solid Liquid And Gas.
From www.numerade.com
SOLVED Which postulate of the theory best accounts Kinetic Theory Of Solid Liquid And Gas The kinetic molecular theory of matter is a concept that basically states that matter is composed of a very large number of very tiny. Gases do not have a fixed volume, and, like liquids, take up the shape of the container. An introduction to kinetic theory. The kinetic molecular theory is a simple but very effective model that effectively explains. Kinetic Theory Of Solid Liquid And Gas.
From teachsimple.com
Theory of Matter Solids, Liquids and Gases, Change of State Kinetic Theory Of Solid Liquid And Gas The theory assumes that gases consist of widely. Gases do not have a fixed volume, and, like liquids, take up the shape of the container. An introduction to kinetic theory. Gases have a very low density. This page takes a simple look at solids, liquids and gases, and changes of state such as melting and boiling, in terms of the. Kinetic Theory Of Solid Liquid And Gas.
From www.yaclass.in
Compressibility of solids, liquids and gases — lesson. Science State Kinetic Theory Of Solid Liquid And Gas Gases have a very low density. This page takes a simple look at solids, liquids and gases, and changes of state such as melting and boiling, in terms of the behaviour of the particles. The kinetic molecular theory of matter is a concept that basically states that matter is composed of a very large number of very tiny. The kinetic. Kinetic Theory Of Solid Liquid And Gas.
From www.shutterstock.com
2,258 Motion Of Particles Of Matter Images, Stock Photos & Vectors Kinetic Theory Of Solid Liquid And Gas This page takes a simple look at solids, liquids and gases, and changes of state such as melting and boiling, in terms of the behaviour of the particles. The kinetic molecular theory is a simple but very effective model that effectively explains ideal gas behavior. The theory assumes that gases consist of widely. Gases do not have a fixed volume,. Kinetic Theory Of Solid Liquid And Gas.
From www.youtube.com
The Theory of Solids YouTube Kinetic Theory Of Solid Liquid And Gas Describe the behavior of an ideal gas. The kinetic molecular theory of matter is a concept that basically states that matter is composed of a very large number of very tiny. This page takes a simple look at solids, liquids and gases, and changes of state such as melting and boiling, in terms of the behaviour of the particles. Study. Kinetic Theory Of Solid Liquid And Gas.
From www.pinterest.com
Question Image Solid liquid gas, States of matter, theory Kinetic Theory Of Solid Liquid And Gas Study how it interacts with gas laws, and view examples. We gain a better understanding of pressure and temperature from the kinetic theory of gases, which assumes that atoms and molecules are in continuous random motion. Gases do not have a fixed volume, and, like liquids, take up the shape of the container. Describe the behavior of an ideal gas.. Kinetic Theory Of Solid Liquid And Gas.
From quizizz.com
50+ solids liquids and gases worksheets for 10th Class on Quizizz Kinetic Theory Of Solid Liquid And Gas An introduction to kinetic theory. Understand kinetic molecular theory and see how it explains the kinetic energy in solids, liquids, & gases. Gases have a very low density. We gain a better understanding of pressure and temperature from the kinetic theory of gases, which assumes that atoms and molecules are in continuous random motion. The kinetic molecular theory of matter. Kinetic Theory Of Solid Liquid And Gas.
From owlcation.com
All About Matter An Introduction to the Basics Owlcation Kinetic Theory Of Solid Liquid And Gas Understand kinetic molecular theory and see how it explains the kinetic energy in solids, liquids, & gases. We gain a better understanding of pressure and temperature from the kinetic theory of gases, which assumes that atoms and molecules are in continuous random motion. The kinetic molecular theory is a simple but very effective model that effectively explains ideal gas behavior.. Kinetic Theory Of Solid Liquid And Gas.
From diagramedia.blogspot.com
Solid Liquid Gas Particles Diagram Diagram Media Kinetic Theory Of Solid Liquid And Gas Describe the behavior of an ideal gas. An introduction to kinetic theory. The theory assumes that gases consist of widely. Understand kinetic molecular theory and see how it explains the kinetic energy in solids, liquids, & gases. Study how it interacts with gas laws, and view examples. The kinetic molecular theory is a simple but very effective model that effectively. Kinetic Theory Of Solid Liquid And Gas.
From studylib.net
The Molecular Theory of Liquids & Solids Kinetic Theory Of Solid Liquid And Gas The kinetic molecular theory is a simple but very effective model that effectively explains ideal gas behavior. The kinetic molecular theory of matter is a concept that basically states that matter is composed of a very large number of very tiny. This page takes a simple look at solids, liquids and gases, and changes of state such as melting and. Kinetic Theory Of Solid Liquid And Gas.
From fyofxukxr.blob.core.windows.net
Solid Liquid And Gas Energy at Winifred Williams blog Kinetic Theory Of Solid Liquid And Gas The kinetic molecular theory of matter is a concept that basically states that matter is composed of a very large number of very tiny. The theory assumes that gases consist of widely. The kinetic molecular theory is a simple but very effective model that effectively explains ideal gas behavior. This page takes a simple look at solids, liquids and gases,. Kinetic Theory Of Solid Liquid And Gas.
From gioigdoqv.blob.core.windows.net
Particles In Solids Liquids And Gases Ks2 at Westberg blog Kinetic Theory Of Solid Liquid And Gas Study how it interacts with gas laws, and view examples. This page takes a simple look at solids, liquids and gases, and changes of state such as melting and boiling, in terms of the behaviour of the particles. Understand kinetic molecular theory and see how it explains the kinetic energy in solids, liquids, & gases. Gases have a very low. Kinetic Theory Of Solid Liquid And Gas.
From gideonkruwcantrell.blogspot.com
Describe Solids Liquids and Gases Using the Theory Kinetic Theory Of Solid Liquid And Gas The kinetic molecular theory of matter is a concept that basically states that matter is composed of a very large number of very tiny. Gases have a very low density. Gases do not have a fixed volume, and, like liquids, take up the shape of the container. Describe the behavior of an ideal gas. We gain a better understanding of. Kinetic Theory Of Solid Liquid And Gas.
From www.sciencelearn.org.nz
model of matter — Science Learning Hub Kinetic Theory Of Solid Liquid And Gas Understand kinetic molecular theory and see how it explains the kinetic energy in solids, liquids, & gases. The theory assumes that gases consist of widely. An introduction to kinetic theory. This page takes a simple look at solids, liquids and gases, and changes of state such as melting and boiling, in terms of the behaviour of the particles. Gases do. Kinetic Theory Of Solid Liquid And Gas.
From www.pinterest.es
Solutions Matter worksheets, States of matter worksheet, Matter science Kinetic Theory Of Solid Liquid And Gas We gain a better understanding of pressure and temperature from the kinetic theory of gases, which assumes that atoms and molecules are in continuous random motion. An introduction to kinetic theory. Gases have a very low density. The kinetic molecular theory of matter is a concept that basically states that matter is composed of a very large number of very. Kinetic Theory Of Solid Liquid And Gas.
From sebschemistry.blogspot.com
IGCSE Edexcel Chemistry Help 1.1 understand the arrangement, movement Kinetic Theory Of Solid Liquid And Gas Study how it interacts with gas laws, and view examples. Understand kinetic molecular theory and see how it explains the kinetic energy in solids, liquids, & gases. The kinetic molecular theory is a simple but very effective model that effectively explains ideal gas behavior. This page takes a simple look at solids, liquids and gases, and changes of state such. Kinetic Theory Of Solid Liquid And Gas.
From cosmosandcrayons.blogspot.com
Cosmos and Crayons Science Facts Thursday The Theory Kinetic Theory Of Solid Liquid And Gas Understand kinetic molecular theory and see how it explains the kinetic energy in solids, liquids, & gases. We gain a better understanding of pressure and temperature from the kinetic theory of gases, which assumes that atoms and molecules are in continuous random motion. Study how it interacts with gas laws, and view examples. The kinetic molecular theory of matter is. Kinetic Theory Of Solid Liquid And Gas.
From www.expii.com
Arrangement of Particles in Phases of Matter — Comparison Expii Kinetic Theory Of Solid Liquid And Gas The kinetic molecular theory is a simple but very effective model that effectively explains ideal gas behavior. Gases do not have a fixed volume, and, like liquids, take up the shape of the container. This page takes a simple look at solids, liquids and gases, and changes of state such as melting and boiling, in terms of the behaviour of. Kinetic Theory Of Solid Liquid And Gas.
From www.englishworksheet.my.id
Molecular Theory Worksheet English Worksheet Kinetic Theory Of Solid Liquid And Gas Gases have a very low density. The kinetic molecular theory is a simple but very effective model that effectively explains ideal gas behavior. The kinetic molecular theory of matter is a concept that basically states that matter is composed of a very large number of very tiny. Study how it interacts with gas laws, and view examples. We gain a. Kinetic Theory Of Solid Liquid And Gas.
From www.youtube.com
The arrangement of particles in solids, liquids and gases Edukite Kinetic Theory Of Solid Liquid And Gas Describe the behavior of an ideal gas. Gases do not have a fixed volume, and, like liquids, take up the shape of the container. The kinetic molecular theory is a simple but very effective model that effectively explains ideal gas behavior. We gain a better understanding of pressure and temperature from the kinetic theory of gases, which assumes that atoms. Kinetic Theory Of Solid Liquid And Gas.
From www.pinterest.com
States of Matter solids, liquids and gases Chemistry for All The Kinetic Theory Of Solid Liquid And Gas Understand kinetic molecular theory and see how it explains the kinetic energy in solids, liquids, & gases. Gases do not have a fixed volume, and, like liquids, take up the shape of the container. The kinetic molecular theory of matter is a concept that basically states that matter is composed of a very large number of very tiny. Study how. Kinetic Theory Of Solid Liquid And Gas.
From www.gasrebate.net
Properties Of Solids Liquids Gases Compared Teachoo Science Kinetic Theory Of Solid Liquid And Gas Study how it interacts with gas laws, and view examples. This page takes a simple look at solids, liquids and gases, and changes of state such as melting and boiling, in terms of the behaviour of the particles. An introduction to kinetic theory. We gain a better understanding of pressure and temperature from the kinetic theory of gases, which assumes. Kinetic Theory Of Solid Liquid And Gas.
From www.visionlearning.com
Properties of Liquids Chemistry Visionlearning Kinetic Theory Of Solid Liquid And Gas We gain a better understanding of pressure and temperature from the kinetic theory of gases, which assumes that atoms and molecules are in continuous random motion. Gases have a very low density. The kinetic molecular theory of matter is a concept that basically states that matter is composed of a very large number of very tiny. The theory assumes that. Kinetic Theory Of Solid Liquid And Gas.
From wordmint.com
Solids,Liquids,Gases Word Search WordMint Kinetic Theory Of Solid Liquid And Gas Study how it interacts with gas laws, and view examples. An introduction to kinetic theory. The kinetic molecular theory is a simple but very effective model that effectively explains ideal gas behavior. Gases do not have a fixed volume, and, like liquids, take up the shape of the container. The theory assumes that gases consist of widely. We gain a. Kinetic Theory Of Solid Liquid And Gas.
From dokumen.tips
(PPT) Solids Liquids and Gas Laws. Theory The three assumptions Kinetic Theory Of Solid Liquid And Gas Understand kinetic molecular theory and see how it explains the kinetic energy in solids, liquids, & gases. The kinetic molecular theory is a simple but very effective model that effectively explains ideal gas behavior. We gain a better understanding of pressure and temperature from the kinetic theory of gases, which assumes that atoms and molecules are in continuous random motion.. Kinetic Theory Of Solid Liquid And Gas.
From blog-0734275gh.blogspot.com
28+ nett Bild Definition Of Solid Matter Define Matter In Science Kinetic Theory Of Solid Liquid And Gas Understand kinetic molecular theory and see how it explains the kinetic energy in solids, liquids, & gases. Study how it interacts with gas laws, and view examples. Gases do not have a fixed volume, and, like liquids, take up the shape of the container. This page takes a simple look at solids, liquids and gases, and changes of state such. Kinetic Theory Of Solid Liquid And Gas.
From vdocuments.mx
· view____6.The theory explains the properties of Kinetic Theory Of Solid Liquid And Gas We gain a better understanding of pressure and temperature from the kinetic theory of gases, which assumes that atoms and molecules are in continuous random motion. An introduction to kinetic theory. Study how it interacts with gas laws, and view examples. This page takes a simple look at solids, liquids and gases, and changes of state such as melting and. Kinetic Theory Of Solid Liquid And Gas.
From sciencetallis.weebly.com
3. Particle Model of Matter THOMAS TALLIS SCIENCE Kinetic Theory Of Solid Liquid And Gas Understand kinetic molecular theory and see how it explains the kinetic energy in solids, liquids, & gases. The kinetic molecular theory is a simple but very effective model that effectively explains ideal gas behavior. An introduction to kinetic theory. Describe the behavior of an ideal gas. Gases have a very low density. Gases do not have a fixed volume, and,. Kinetic Theory Of Solid Liquid And Gas.
From igcsechemistryrevision.weebly.com
1.1 Understand the arrangement, movement and energy of particles in Kinetic Theory Of Solid Liquid And Gas This page takes a simple look at solids, liquids and gases, and changes of state such as melting and boiling, in terms of the behaviour of the particles. The kinetic molecular theory of matter is a concept that basically states that matter is composed of a very large number of very tiny. Understand kinetic molecular theory and see how it. Kinetic Theory Of Solid Liquid And Gas.
From www.tes.com
theory of solids, liquids and gases Teaching Resources Kinetic Theory Of Solid Liquid And Gas Gases have a very low density. The kinetic molecular theory is a simple but very effective model that effectively explains ideal gas behavior. The kinetic molecular theory of matter is a concept that basically states that matter is composed of a very large number of very tiny. Study how it interacts with gas laws, and view examples. Gases do not. Kinetic Theory Of Solid Liquid And Gas.