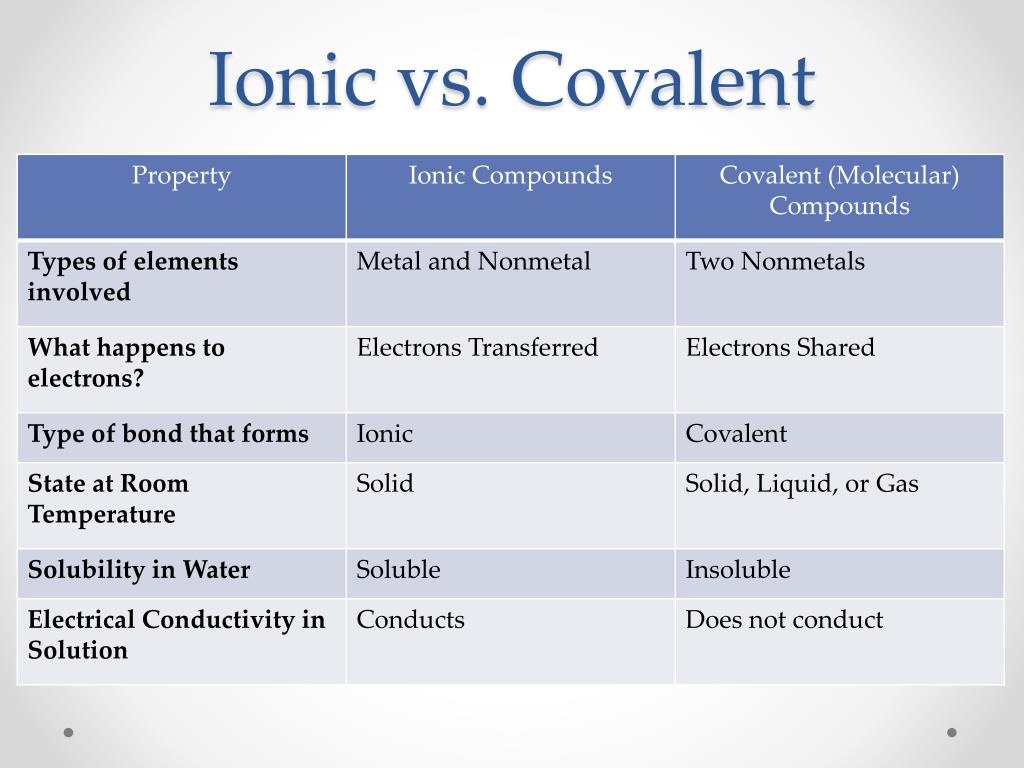
What Makes Ionic And Covalent Bonds Different . Ionic bonds are formed when one ion — an atom or molecule with a net charge, either positive or negative — finds another ion of the opposite charge to bond with, creating an. Compounds that contain covalent bonds exhibit different physical properties than ionic compounds. The reaction components of covalent bonds are electrically neutral,. In covalent bonds, atoms share electrons, whereas in ionic bonds atoms transfer electrons. In ionic compounds all the ions are. Ionic bonds require at least one electron donor and one electron acceptor. They differ in their structure and properties. They differ in their structure and properties. Covalent bonds consist of pairs. Covalent bonds consist of pairs of electrons shared by two atoms,. The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic bond while electrons are shared between. In ionic bonding, atoms transfer electrons to each other.
from www.slideserve.com
Ionic bonds are formed when one ion — an atom or molecule with a net charge, either positive or negative — finds another ion of the opposite charge to bond with, creating an. In ionic compounds all the ions are. The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic bond while electrons are shared between. In ionic bonding, atoms transfer electrons to each other. Covalent bonds consist of pairs. They differ in their structure and properties. Covalent bonds consist of pairs of electrons shared by two atoms,. They differ in their structure and properties. Ionic bonds require at least one electron donor and one electron acceptor. In covalent bonds, atoms share electrons, whereas in ionic bonds atoms transfer electrons.
PPT Chemical Bonds (Ionic and Covalent Bonds) Part 1 PowerPoint
What Makes Ionic And Covalent Bonds Different Covalent bonds consist of pairs. They differ in their structure and properties. Ionic bonds require at least one electron donor and one electron acceptor. In ionic bonding, atoms transfer electrons to each other. Ionic bonds are formed when one ion — an atom or molecule with a net charge, either positive or negative — finds another ion of the opposite charge to bond with, creating an. The reaction components of covalent bonds are electrically neutral,. Covalent bonds consist of pairs. In ionic compounds all the ions are. The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic bond while electrons are shared between. They differ in their structure and properties. Covalent bonds consist of pairs of electrons shared by two atoms,. Compounds that contain covalent bonds exhibit different physical properties than ionic compounds. In covalent bonds, atoms share electrons, whereas in ionic bonds atoms transfer electrons.
From www.youtube.com
Ionic and Covalent Bonding Chemistry YouTube What Makes Ionic And Covalent Bonds Different Compounds that contain covalent bonds exhibit different physical properties than ionic compounds. In ionic bonding, atoms transfer electrons to each other. The reaction components of covalent bonds are electrically neutral,. In covalent bonds, atoms share electrons, whereas in ionic bonds atoms transfer electrons. Covalent bonds consist of pairs. Ionic bonds are formed when one ion — an atom or molecule. What Makes Ionic And Covalent Bonds Different.
From www.masterorganicchemistry.com
Ionic and Covalent Bonding Master Organic Chemistry What Makes Ionic And Covalent Bonds Different The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic bond while electrons are shared between. Compounds that contain covalent bonds exhibit different physical properties than ionic compounds. In covalent bonds, atoms share electrons, whereas in ionic bonds atoms transfer electrons. In ionic compounds all the ions. What Makes Ionic And Covalent Bonds Different.
From pediaa.com
Difference Between Covalent and Ionic Bonds What Makes Ionic And Covalent Bonds Different In covalent bonds, atoms share electrons, whereas in ionic bonds atoms transfer electrons. The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic bond while electrons are shared between. The reaction components of covalent bonds are electrically neutral,. In ionic compounds all the ions are. Compounds that. What Makes Ionic And Covalent Bonds Different.
From www.studocu.com
Chemical Bondinganswers Chemical Bonding Worksheet Ionic Bond What Makes Ionic And Covalent Bonds Different Compounds that contain covalent bonds exhibit different physical properties than ionic compounds. They differ in their structure and properties. They differ in their structure and properties. In ionic bonding, atoms transfer electrons to each other. Ionic bonds are formed when one ion — an atom or molecule with a net charge, either positive or negative — finds another ion of. What Makes Ionic And Covalent Bonds Different.
From www.pinterest.co.uk
Difference between ionic ,covalent and coordinate covalent bond What Makes Ionic And Covalent Bonds Different In ionic compounds all the ions are. Covalent bonds consist of pairs. In covalent bonds, atoms share electrons, whereas in ionic bonds atoms transfer electrons. Ionic bonds are formed when one ion — an atom or molecule with a net charge, either positive or negative — finds another ion of the opposite charge to bond with, creating an. Compounds that. What Makes Ionic And Covalent Bonds Different.
From ar.inspiredpencil.com
Covalent Bond Examples List What Makes Ionic And Covalent Bonds Different Compounds that contain covalent bonds exhibit different physical properties than ionic compounds. They differ in their structure and properties. They differ in their structure and properties. In covalent bonds, atoms share electrons, whereas in ionic bonds atoms transfer electrons. Covalent bonds consist of pairs. Ionic bonds require at least one electron donor and one electron acceptor. In ionic compounds all. What Makes Ionic And Covalent Bonds Different.
From www.slideserve.com
PPT Chemical Bonds (Ionic and Covalent Bonds) Part 1 PowerPoint What Makes Ionic And Covalent Bonds Different The reaction components of covalent bonds are electrically neutral,. In ionic compounds all the ions are. Covalent bonds consist of pairs. In ionic bonding, atoms transfer electrons to each other. They differ in their structure and properties. Ionic bonds are formed when one ion — an atom or molecule with a net charge, either positive or negative — finds another. What Makes Ionic And Covalent Bonds Different.
From www.slideserve.com
PPT The Structure of Matter PowerPoint Presentation, free download What Makes Ionic And Covalent Bonds Different Ionic bonds require at least one electron donor and one electron acceptor. In ionic bonding, atoms transfer electrons to each other. In ionic compounds all the ions are. They differ in their structure and properties. Ionic bonds are formed when one ion — an atom or molecule with a net charge, either positive or negative — finds another ion of. What Makes Ionic And Covalent Bonds Different.
From ar.inspiredpencil.com
Covalent Bonding Vs Ionic Bonding What Makes Ionic And Covalent Bonds Different Covalent bonds consist of pairs of electrons shared by two atoms,. The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic bond while electrons are shared between. In ionic compounds all the ions are. In covalent bonds, atoms share electrons, whereas in ionic bonds atoms transfer electrons.. What Makes Ionic And Covalent Bonds Different.
From thisonevsthatone.com
Ionic vs Covalent Which is which and how to tell them apart What Makes Ionic And Covalent Bonds Different Covalent bonds consist of pairs. In covalent bonds, atoms share electrons, whereas in ionic bonds atoms transfer electrons. They differ in their structure and properties. The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic bond while electrons are shared between. Ionic bonds are formed when one. What Makes Ionic And Covalent Bonds Different.
From educational.my.id
Ionic And Covalent Bonds Worksheet Educational.my.id What Makes Ionic And Covalent Bonds Different They differ in their structure and properties. They differ in their structure and properties. The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic bond while electrons are shared between. Covalent bonds consist of pairs of electrons shared by two atoms,. The reaction components of covalent bonds. What Makes Ionic And Covalent Bonds Different.
From mungfali.com
Ionic And Covalent Bonds Venn Diagram What Makes Ionic And Covalent Bonds Different In covalent bonds, atoms share electrons, whereas in ionic bonds atoms transfer electrons. They differ in their structure and properties. Covalent bonds consist of pairs. The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic bond while electrons are shared between. The reaction components of covalent bonds. What Makes Ionic And Covalent Bonds Different.
From www.logiota.com
Ionic and Covalent Bond Chemical Bonding Physical Chemistry What Makes Ionic And Covalent Bonds Different In ionic compounds all the ions are. They differ in their structure and properties. In ionic bonding, atoms transfer electrons to each other. Covalent bonds consist of pairs of electrons shared by two atoms,. In covalent bonds, atoms share electrons, whereas in ionic bonds atoms transfer electrons. They differ in their structure and properties. Covalent bonds consist of pairs. Ionic. What Makes Ionic And Covalent Bonds Different.
From nethenncowen.blogspot.com
Difference Between Ionic and Covalent Bonds NethennCowen What Makes Ionic And Covalent Bonds Different They differ in their structure and properties. Ionic bonds require at least one electron donor and one electron acceptor. Compounds that contain covalent bonds exhibit different physical properties than ionic compounds. Ionic bonds are formed when one ion — an atom or molecule with a net charge, either positive or negative — finds another ion of the opposite charge to. What Makes Ionic And Covalent Bonds Different.
From infogram.com
Covalent Bonds .VS. Ionic Bonds Infogram What Makes Ionic And Covalent Bonds Different The reaction components of covalent bonds are electrically neutral,. In ionic compounds all the ions are. Covalent bonds consist of pairs of electrons shared by two atoms,. The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic bond while electrons are shared between. They differ in their. What Makes Ionic And Covalent Bonds Different.
From quizlet.com
Ionic and Covalent Bonds Diagram Quizlet What Makes Ionic And Covalent Bonds Different Compounds that contain covalent bonds exhibit different physical properties than ionic compounds. The reaction components of covalent bonds are electrically neutral,. They differ in their structure and properties. In ionic bonding, atoms transfer electrons to each other. They differ in their structure and properties. In ionic compounds all the ions are. Ionic bonds require at least one electron donor and. What Makes Ionic And Covalent Bonds Different.
From sciencenotes.org
Covalent Compounds Examples and Properties What Makes Ionic And Covalent Bonds Different Covalent bonds consist of pairs of electrons shared by two atoms,. Compounds that contain covalent bonds exhibit different physical properties than ionic compounds. Covalent bonds consist of pairs. They differ in their structure and properties. In ionic bonding, atoms transfer electrons to each other. They differ in their structure and properties. Ionic bonds are formed when one ion — an. What Makes Ionic And Covalent Bonds Different.
From sciencenotes.org
Ionic vs Covalent Bonds What Makes Ionic And Covalent Bonds Different They differ in their structure and properties. In ionic compounds all the ions are. The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic bond while electrons are shared between. They differ in their structure and properties. The reaction components of covalent bonds are electrically neutral,. In. What Makes Ionic And Covalent Bonds Different.
From www.expii.com
Ionic Bonding (Biology) — Definition & Role Expii What Makes Ionic And Covalent Bonds Different Ionic bonds are formed when one ion — an atom or molecule with a net charge, either positive or negative — finds another ion of the opposite charge to bond with, creating an. In ionic compounds all the ions are. Covalent bonds consist of pairs of electrons shared by two atoms,. Ionic bonds require at least one electron donor and. What Makes Ionic And Covalent Bonds Different.
From www.youtube.com
Ionic bonds vs Covalent bonds explained. YouTube What Makes Ionic And Covalent Bonds Different They differ in their structure and properties. The reaction components of covalent bonds are electrically neutral,. Covalent bonds consist of pairs. Ionic bonds require at least one electron donor and one electron acceptor. They differ in their structure and properties. The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom. What Makes Ionic And Covalent Bonds Different.
From spixasil.blogspot.com
Covalent Bond Is Between Covalent Bonds vs. Ionic Bonds What is The What Makes Ionic And Covalent Bonds Different Covalent bonds consist of pairs of electrons shared by two atoms,. In ionic bonding, atoms transfer electrons to each other. In covalent bonds, atoms share electrons, whereas in ionic bonds atoms transfer electrons. Compounds that contain covalent bonds exhibit different physical properties than ionic compounds. Ionic bonds are formed when one ion — an atom or molecule with a net. What Makes Ionic And Covalent Bonds Different.
From gianniabvargas.blogspot.com
Ionic Bond Vs Covalent Bond romantic johnny cash quotes love What Makes Ionic And Covalent Bonds Different They differ in their structure and properties. Ionic bonds are formed when one ion — an atom or molecule with a net charge, either positive or negative — finds another ion of the opposite charge to bond with, creating an. Covalent bonds consist of pairs of electrons shared by two atoms,. Covalent bonds consist of pairs. The reaction components of. What Makes Ionic And Covalent Bonds Different.
From www.teachoo.com
[Class 10] Differentiate between Ionic bond & Covalent bond Teachoo What Makes Ionic And Covalent Bonds Different The reaction components of covalent bonds are electrically neutral,. In ionic compounds all the ions are. Compounds that contain covalent bonds exhibit different physical properties than ionic compounds. Ionic bonds require at least one electron donor and one electron acceptor. In covalent bonds, atoms share electrons, whereas in ionic bonds atoms transfer electrons. Covalent bonds consist of pairs. In ionic. What Makes Ionic And Covalent Bonds Different.
From www.differencebetween.com
Difference Between Ionic and Covalent Bonds Compare the Difference What Makes Ionic And Covalent Bonds Different They differ in their structure and properties. Compounds that contain covalent bonds exhibit different physical properties than ionic compounds. Ionic bonds are formed when one ion — an atom or molecule with a net charge, either positive or negative — finds another ion of the opposite charge to bond with, creating an. In ionic compounds all the ions are. In. What Makes Ionic And Covalent Bonds Different.
From www.jagranjosh.com
What Is The Difference Between Ionic And Covalent Bonds? What Makes Ionic And Covalent Bonds Different Compounds that contain covalent bonds exhibit different physical properties than ionic compounds. The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic bond while electrons are shared between. Ionic bonds require at least one electron donor and one electron acceptor. They differ in their structure and properties.. What Makes Ionic And Covalent Bonds Different.
From pediaa.com
Difference Between Ionic Covalent and Metallic Bonds Definition What Makes Ionic And Covalent Bonds Different Covalent bonds consist of pairs. Compounds that contain covalent bonds exhibit different physical properties than ionic compounds. In ionic bonding, atoms transfer electrons to each other. Ionic bonds are formed when one ion — an atom or molecule with a net charge, either positive or negative — finds another ion of the opposite charge to bond with, creating an. They. What Makes Ionic And Covalent Bonds Different.
From sciencenotes.org
Compounds With Both Ionic and Covalent Bonds What Makes Ionic And Covalent Bonds Different Covalent bonds consist of pairs of electrons shared by two atoms,. Ionic bonds are formed when one ion — an atom or molecule with a net charge, either positive or negative — finds another ion of the opposite charge to bond with, creating an. Compounds that contain covalent bonds exhibit different physical properties than ionic compounds. In ionic bonding, atoms. What Makes Ionic And Covalent Bonds Different.
From examples.yourdictionary.com
Main Differences Between Ionic and Covalent Bonds YourDictionary What Makes Ionic And Covalent Bonds Different Ionic bonds require at least one electron donor and one electron acceptor. In ionic compounds all the ions are. In ionic bonding, atoms transfer electrons to each other. Ionic bonds are formed when one ion — an atom or molecule with a net charge, either positive or negative — finds another ion of the opposite charge to bond with, creating. What Makes Ionic And Covalent Bonds Different.
From www.expii.com
Ionic Bonding (Biology) — Definition & Role Expii What Makes Ionic And Covalent Bonds Different The reaction components of covalent bonds are electrically neutral,. Ionic bonds require at least one electron donor and one electron acceptor. Compounds that contain covalent bonds exhibit different physical properties than ionic compounds. Ionic bonds are formed when one ion — an atom or molecule with a net charge, either positive or negative — finds another ion of the opposite. What Makes Ionic And Covalent Bonds Different.
From pediaa.com
Difference Between Ionic Covalent and Metallic Bonds Definition What Makes Ionic And Covalent Bonds Different In ionic bonding, atoms transfer electrons to each other. They differ in their structure and properties. They differ in their structure and properties. Compounds that contain covalent bonds exhibit different physical properties than ionic compounds. Ionic bonds are formed when one ion — an atom or molecule with a net charge, either positive or negative — finds another ion of. What Makes Ionic And Covalent Bonds Different.
From wou.edu
CH104 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry What Makes Ionic And Covalent Bonds Different In ionic compounds all the ions are. They differ in their structure and properties. Covalent bonds consist of pairs. They differ in their structure and properties. Compounds that contain covalent bonds exhibit different physical properties than ionic compounds. The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an. What Makes Ionic And Covalent Bonds Different.
From www.studocu.com
Lab Report Ionic and Covalent Bonds (21) NAME DATE Lab Report Ionic What Makes Ionic And Covalent Bonds Different They differ in their structure and properties. The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic bond while electrons are shared between. Covalent bonds consist of pairs. Ionic bonds are formed when one ion — an atom or molecule with a net charge, either positive or. What Makes Ionic And Covalent Bonds Different.
From www.youtube.com
How to tell if Ionic Bond or Covalent Bond YouTube What Makes Ionic And Covalent Bonds Different Covalent bonds consist of pairs. The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic bond while electrons are shared between. They differ in their structure and properties. In covalent bonds, atoms share electrons, whereas in ionic bonds atoms transfer electrons. Covalent bonds consist of pairs of. What Makes Ionic And Covalent Bonds Different.
From coredifferences.com
15 Major Difference between Covalent and Ionic Bonds with Table Core What Makes Ionic And Covalent Bonds Different The reaction components of covalent bonds are electrically neutral,. In covalent bonds, atoms share electrons, whereas in ionic bonds atoms transfer electrons. In ionic compounds all the ions are. Covalent bonds consist of pairs. Ionic bonds are formed when one ion — an atom or molecule with a net charge, either positive or negative — finds another ion of the. What Makes Ionic And Covalent Bonds Different.
From www.youtube.com
Metallic, Ionic, and Molecular Solids Explained with What Makes Ionic And Covalent Bonds Different The key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic bond while electrons are shared between. Covalent bonds consist of pairs. Ionic bonds require at least one electron donor and one electron acceptor. They differ in their structure and properties. Compounds that contain covalent bonds exhibit different. What Makes Ionic And Covalent Bonds Different.